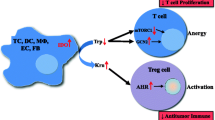
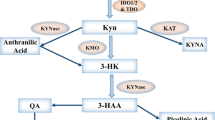
Abstract
Cancer immunotherapy utilizes the immune system and its wide-ranging components to deliver anti-tumor responses. In immune escape mechanisms, tumor microenvironment-associated soluble factors and cell surface-bound molecules are mainly accountable for the dysfunctional activity of tumor-specific CD8+ T cells, natural killer (NK) cells, tumor associated macrophages (TAMs) and stromal cells. The myeloid-derived suppressor cells (MDSCs) and Foxp3+ regulatory T cells (Tregs), are also key tumor-promoting immune cells. These potent immunosuppressive networks avert tumor rejection at various stages, affecting immunotherapies' outcomes. Numerous clinical trials have elucidated that disruption of immunosuppression could be achieved via checkpoint inhibitors. Another approach utilizes enzymes that can restore the body’s potential to counter cancer by triggering the immune system inhibited by the tumor microenvironment. These immunotherapeutic enzymes can catalyze an immunostimulatory signal and modulate the tumor microenvironment via effector molecules. Herein, we have discussed the immuno-metabolic roles of various enzymes like ATP-dephosphorylating ectoenzymes, inducible Nitric Oxide Synthase, phenylamine, tryptophan, and arginine catabolizing enzymes in cancer immunotherapy. Understanding the detailed molecular mechanisms of the enzymes involved in modulating the tumor microenvironment may help find new opportunities for cancer therapeutics.


Similar content being viewed by others
Data availability
Not applicable.
References
Sung F, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;71:209–49. https://doi.org/10.3322/caac.21660.
Coley WB. Contribution to the knowledge of Sarcoma. Ann Surg. 1891;14:199–220. https://doi.org/10.1097/00000658-189112000-00015.
Burnet M. Cancer-A biological approach: III. viruses associated with neoplastic conditions. IV. practical applications. BMJ. 1957. https://doi.org/10.1136/bmj.1.5023.841.
Thomas L, Lawrence HS. Cellular and humoral aspects of the hypersensitive states. New York, NY, USA: Hoeber-Harper; 1959.
Khan M, Maker AV, Jain S. The Evolution of cancer immunotherapy. Vaccines (Basel). 2021;9(6):614. https://doi.org/10.3390/vaccines9060614.
Stanley M. Tumour virus vaccines: Hepatitis B virus and human papillomavirus. Trans R Soc B Biol Sci Philos. 2017. https://doi.org/10.1098/rstb.2016.0268.
Berraondo P, Sanmamed MF, Ochoa MC, et al. Cytokines in clinical cancer immunotherapy. British J Cancer. 2019;120:6–15. https://doi.org/10.1038/s41416-018-0328-y.
Conry RM, Westbrook B, McKee S, et al. Talimogene laherparepvec: first in class oncolytic virotherapy. Hum Vaccines Immunother. 2018;14:839–46. https://doi.org/10.1080/21645515.2017.1412896.
Miliotou AN, Papadopoulou LC. CAR T-cell therapy: a new era in cancer immunotherapy. Curr Pharm Biotechnol. 2018;19:5–18. https://doi.org/10.2174/1389201019666180418095526.
Wei SC, Anang NA, Sharma R, et al. Combination anti–CTLA-4 plus anti–PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci. 2019;116:22699–709. https://doi.org/10.1073/pnas.1821218116.
Popp FC, Capino I, Bartels J, et al. Expression of immune checkpoint regulators IDO, VISTA, LAG3, and TIM3 in resected pancreatic ductal adenocarcinoma. Cancers. 2021;13:2689. https://doi.org/10.3390/cancers13112689.
Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin cancer biol. 2015;35:S185–98. https://doi.org/10.1016/j.semcancer.2015.03.004.
Guerrouahen BS, Maccalli C, Cugno C, et al. Reverting immune suppression to enhance cancer immunotherapy. Front onco. 2020;9:1554. https://doi.org/10.3389/fonc.2019.01554.
Wilson EB, El-Jawhari JJ, Neilson AL, et al. Human tumour immune evasion Via TGF-b Blocks Nk Cell activation but not survival allowing therapeutic restoration of anti-tumour activity. PLoS ONE. 2011;6: e22842. https://doi.org/10.1371/journal.pone.0022842.
Rooney MS, Shukla SA, Wu CJ, et al. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. https://doi.org/10.1016/j.cell.2014.12.033.
Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol. 2020;11:940. https://doi.org/10.3389/fimmu.2020.00940.
Ugel S, De Sanctis F, Mandruzzato S, et al. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J Clin Investig. 2015;125:3365–76. https://doi.org/10.1172/JCI80006.
Baghban R, Roshangar L, Jahanban-Esfahlan R. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18:1–19. https://doi.org/10.1186/s12964-020-0530-4.
Gold B, Cankovic M, Furtado LV, et al. Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility?: a report of the association for molecular pathology. J Mol Diagn. 2015;17:209–24. https://doi.org/10.1016/j.jmoldx.2015.02.001.
Mansoori B, Mohammadi A, Davudian S, et al. The different mechanisms of cancer drug resistance: a brief review. Pharm Bull Adv. 2017. https://doi.org/10.5171/apb.2017.041.
Wu L, Qu X. Cancer biomarker detection: recent achievements and challenges. Chem Soc Rev. 2015;44:2963–97. https://doi.org/10.1039/c4cs00370e.
Molinier-Frenkel V, Castellano F. Immunosuppressive enzymes in the tumor microenvironment. FEBS Lett. 2017;591:3135–57. https://doi.org/10.1002/1873-3468.12784.
Lemos H, Huang L, Prendergast GC, et al. Immune control by amino acid catabolism during tumorigenesis and therapy. Nat Rev Cancer. 2019;19:162–75. https://doi.org/10.1038/s41568-019-0106-z.
Lemberg KM, Gori SS, Tsukamoto T, Rais R, Slusher BS. Clinical development of metabolic inhibitors for oncology. J Clin Invest. 2022;132: e148550. https://doi.org/10.1172/jci148550.
Molinier-Frenkel V, Prévost-Blondel A, Castellano F. The IL4I1 enzyme: a new player in the immunosuppressive tumor microenvironment. Cells. 2019;8:757. https://doi.org/10.3390/cells8070757.
Santarlasci V, Maggi L, Mazzoni A, et al. IL-4-induced gene 1 maintains high Tob1 expression that contributes to TCR unresponsiveness in human T helper 17 cells. Eur J Immunol. 2014;44:654–61. https://doi.org/10.1002/eji.201344047.
Boulland ML, Marquet J, Molinier-Frenkel V, et al. Human IL4I1 is a secreted L-phenylalanine oxidase expressed by mature dendritic cells that inhibits T-lymphocyte proliferation. Blood. 2007;110:220–7. https://doi.org/10.1182/blood-2006-07-036210.
Maier B, Leader AM, Chen ST, et al. A conserved dendritic-cell regulatory program limits antitumour immunity. Nature. 2020;580:257–62. https://doi.org/10.1038/s41586-020-2134-y.
Liu Y, He S, Wang X-L, et al. Tumour heterogeneity and intercellular networks of nasopharyngeal carcinoma at single cell resolution. Nat Commun. 2021. https://doi.org/10.1038/s41467-021-21043-4.
Zhao H, Teng Y, Hao W, et al. Single-cell analysis revealed that IL4I1 promoted ovarian cancer progression. J Transl Med. 2021;19:454. https://doi.org/10.1186/s12967-021-03123-7.
Marquet J, Lasoudris F, Cousin C, et al. Dichotomy between factors inducing the immunosuppressive enzyme IL4I1 in B lymphocytes and mononuclear phagocytes. Eur J Immunol. 2010;40:2557–68. https://doi.org/10.1002/eji.201040428.
Carbonnelle-Puscian A, Copie-Bergman C, Baia M, et al. The novel immunosuppressive enzyme IL4I1 is expressed by neoplastic cells of several B-cell lymphomas and by tumor-associated macrophages. Leukemia. 2009;23:952–60. https://doi.org/10.1038/leu.2008.380.
Lasoudris F, Cousin C, Prevost-Blondel A, et al. IL4I1: an inhibitor of the CD8(+) antitumor T cell response in vivo. Eur J Immunol. 2011;41:1629–38. https://doi.org/10.1002/eji.201041119.
Choueiry F, Singh S, Sircar A, et al. Integration of metabolomics and gene expression profiling elucidates IL4I1 as modulator of Ibrutinib resistance in ABC-diffuse large B Cell lymphoma. Cancers. 2021;13:2146. https://doi.org/10.3390/cancers13092146.
Bod L, Lengagne R, Wrobel L, et al. IL4-induced gene 1 promotes tumor growth by shaping the immune microenvironment in melanoma. Oncoimmunol. 2017;6: e1278331. https://doi.org/10.1080/2162402x.2016.1278331.
Sadik A, Patterson LF, Öztürk S, et al. IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell. 2020;182:1252–70. https://doi.org/10.1016/j.cell.2020.07.038.
Rao D, Yu C, Wang T, et al. Pan-cancer analysis combined with experimental validation revealed IL4I1 as an immunological and prognostic biomarker. Int Immunopharmacol. 2022;111: 109091. https://doi.org/10.1016/j.intimp.2022.109091.
Mazzoni A, Capone M, Ramazzotti M, et al. IL4I1 Is expressed by head-neck cancer-derived mesenchymal stromal cells and contributes to suppress T Cell proliferation. J Clin Med. 2021;10:2111. https://doi.org/10.3390/jcm10102111.
Wu D, Zhu Y. Role of kynurenine in promoting the generation of exhausted CD8+ T cells in colorectal cancer. Am J Transl Res. 2021;13:1535.
Holmgaard RB, Zamarin D, Li Y, et al. Tumor-expressed IDO recruits and activates MDSCs in a treg-dependent manner. Cell Rep. 2015;13:412–24. https://doi.org/10.1016/j.celrep.2015.08.077.
Terrén I, Orrantia A, Vitallé J, et al. NK cell metabolism and tumor microenvironment. Front Immunol. 2019;10:2278. https://doi.org/10.3389/fimmu.2019.02278.
Sullivan MR, Danai LV, Lewis CA, et al. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. Elife. 2019;8: e44235. https://doi.org/10.7554/eLife.44235.
Wang D, Saga Y, Mizukami H, et al. Indoleamine-2, 3-dioxygenase, an immunosuppressive enzyme that inhibits natural killer cell function, as a useful target for ovarian cancer therapy. Int J Oncol. 2012;40:929–34. https://doi.org/10.3892/ijo.2011.1295.
Wei L, Zhu S, Li M, et al. High indoleamine 2,3-dioxygenase is correlated with microvessel density and worse prognosis in breast cancer. Front Immunol. 2018;9:724. https://doi.org/10.3389/fimmu.2018.00724.
Kim D, Kim JM, Kim JS, et al. Differential expression and clinicopathological significance of HER2, indoleamine 2,3-dioxygenase and PD-L1 in urothelial carcinoma of the bladder. J Clin Med. 2020;9:1265. https://doi.org/10.3390/jcm9051265.
Meireson A, Chevolet I, Hulstaert E, et al. Peritumoral endothelial indoleamine 2, 3-dioxygenase expression is an early independent marker of disease relapse in colorectal cancer and is influenced by DNA mismatch repair profile. Oncotarget. 2018. https://doi.org/10.18632/oncotarget.25393.
Löb S, Königsrainer A, Zieker D, et al. IDO1 and IDO2 are expressed in human tumors: levo-but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer immunol Immunother. 2009;58:153–7. https://doi.org/10.1007/s00262-008-0513-6.
Muller AJ, DuHadaway JB, Donover PS, et al. Inhibition of indoleamine 2, 3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–9. https://doi.org/10.1038/nm1196.
Jia Y, Wang H, Wang Y, et al. Low expression of Bin1, along with high expression of IDO in tumor tissue and draining lymph nodes, are predictors of poor prognosis for esophageal squamous cell cancer patients. Int J Cancer. 2015;137:1095–106. https://doi.org/10.1002/ijc.29481.
Salvadori ML, da Cunha Bianchi PK, et al. Effect of the association of 1-methyl-DL-tryptophan with paclitaxel on the expression of indoleamine 2,3-dioxygenase in cultured cancer cells from patients with breast cancer. Med Oncol. 2015;32:248. https://doi.org/10.1007/s12032-015-0694-8.
Newman AC, Falcone M, Uribe AH, et al. Immune-regulated IDO1-dependent tryptophan metabolism is source of one-carbon units for pancreatic cancer and stellate cells. Mol cell. 2021;81:2290–302. https://doi.org/10.1016/j.molcel.2021.03.019.
Zhang X, Liu X, Zhou W, et al. Blockade of IDO-kynurenine-AhR axis ameliorated colitis-associated colon cancer via inhibiting immune tolerance. Cell Mol Gastroenterol Hepatol. 2021;12:1179–99. https://doi.org/10.1016/j.jcmgh.2021.05.018.
Holmgaard RB, Zamarin D, Munn DH, et al. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med. 2013;210:1389–402. https://doi.org/10.1084/jem.20130066.
Shang K, Wang Z, Hu Y, et al. Gene silencing of indoleamine 2, 3-dioxygenase 1 inhibits lung cancer growth by suppressing T cell exhaustion. Oncol Lett. 2020;19:3827–38. https://doi.org/10.3892/ol.2020.11477.
Endo R, Nakamura T, Kawakami K, et al. The silencing of indoleamine 2, 3-dioxygenase 1 (IDO1) in dendritic cells by siRNA-loaded lipid nanoparticles enhances cell-based cancer immunotherapy. Sci. 2019;9:1–11. https://doi.org/10.1038/s41598-019-47799-w.
Pan J, Yuan K, Peng S, et al. Gene silencing of indoleamine 2, 3-dioxygenase hinders tumor growth through angiogenesis inhibition. Int J oncol. 2017;50:2136–44. https://doi.org/10.3892/ijo.2017.3975.
Cui G, Li C, Xu G, et al. Tumor-associated fibroblasts and microvessels contribute to the expression of immunosuppressive factor indoleamine 2, 3-dioxygenase in human esophageal cancers. Pathol Oncol Res. 2018;24:269–75. https://doi.org/10.1007/s12253-017-0244-0.
Ling W, Zhang J, Yuan Z, et al. Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res. 2014;74:1576–87. https://doi.org/10.1158/0008-5472.can-13-1656.
Jaufmann J, Lelis FJN, Teschner AC, et al. Human monocytic myeloid-derived suppressor cells impair B cell phenotype and function in vitro. Eur J Immunol. 2020;50:33–47. https://doi.org/10.1002/eji.201948240.
Liu Y, Liang X, Yin X, et al. Blockade of IDO-kynurenine-AhR metabolic circuitry abrogates IFN-γ-induced immunologic dormancy of tumor-repopulating cells. Nat Commun. 2017. https://doi.org/10.1038/ncomms15207.
Liu M, Wang X, Wang L, et al. Targeting the IDO1 pathway in cancer: from bench to bedside. J hematol oncol. 2018;11:1–12. https://doi.org/10.1186/s13045-018-0644-y.
Beatty GL, O’Dwyer PJ, Clark J, et al. First-in-human phase I study of the oral inhibitor of indoleamine 2,3-dioxygenase-1 epacadostat (INCB024360) in patients with advanced solid malignancies. Clin Cancer Res. 2017;23:3269–76. https://doi.org/10.1158/1078-0432.ccr-16-2272.
Jung KH, LoRusso P, Burris H, et al. Phase I study of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) administered with PD-L1 inhibitor (atezolizumab) in advanced solid tumors. Clin Cancer Res. 2019;25:3220–8. https://doi.org/10.1158/1078-0432.ccr-18-2740.
Siu LL, Gelmon K, Chu Q, et al. Abstract CT116: BMS-986205, an optimized indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor, is well tolerated with potent pharmacodynamic (PD) activity, alone and in combination with nivolumab (nivo) in advanced cancers in a phase 1/2a trial. Cancer Res. 2017. https://doi.org/10.1158/1538-7445.AM2017-CT116.
Spranger S, Koblish HK, Horton B, et al. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8+ T cells directly within the tumor microenvironment. J Immunother Cancer. 2014;2:1–4. https://doi.org/10.1186/2051-1426-2-3.
Long GV, Dummer R, Hamid O, et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20:1083–97. https://doi.org/10.1016/S1470-2045(19)30274-8.
Park A, Yang Y, Lee Y, Kim MS, Park Y-J, Jung H, Kim T-D, Lee HG, Choi I, Yoon SR. Indoleamine-2, 3-dioxygenase in thyroid cancer cells suppresses natural killer cell function by inhibiting NKG2D and NKp46 expression via STAT signaling pathways. J Clin Med. 2019. https://doi.org/10.3390/jcm8060842.
Zhang J, Han X, Hu X, Jin F, Gao Z, Yin L, Qin J, Yin F, Li C, Wang Y. IDO1 impairs NK cell cytotoxicity by decreasing NKG2D/NKG2DLs via promoting miR-18a. Mol Immunol. 2018;103:144–55. https://doi.org/10.1016/j.molimm.2018.09.011.
Yang SL, Niu THX, et al. The IFN-gamma-IDO1-kynureine pathway-induced autophagy in cervical cancer cell promotes phagocytosis of macrophage. Int J Biol Sci. 2021. https://doi.org/10.7150/ijbs.51241.
Fatokun AA, Hunt NH, Ball HJ. Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease. Amino Acids. 2013;45:1319–29. https://doi.org/10.1007/s00726-013-1602-1.
Ball HJ, Sanchez-Perez A, Weiser S, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396:203–13. https://doi.org/10.1016/j.gene.2007.04.010.
Qian F, Liao J, Villella J, et al. Effects of 1-methyltryptophan stereoisomers on IDO2 enzyme activity and IDO2-mediated arrest of human T cell proliferation. Cancer Immunol Immunother. 2012;61:2013–20. https://doi.org/10.1007/s00262-012-1265-x.
Yamasuge W, Yamamoto Y, Fujigaki H, et al. Indoleamine 2,3-dioxygenase 2 depletion suppresses tumor growth in a mouse model of Lewis lung carcinoma. Cancer Sci. 2019;110:3061–7. https://doi.org/10.1111/cas.14179.
Nevler A, Muller AJ, Sutanto-Ward E, et al. Host IDO2 gene status influences tumor progression and radiotherapy response in KRAS driven sporadic pancreatic cancers. Clin Cancer Res. 2019;25:724–34. https://doi.org/10.1158/1078-0432.ccr-18-0814.
Witkiewicz AK, Costantino CL, Metz R, et al. Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel active target. J Am Coll Surg. 2009. https://doi.org/10.1016/j.jamcollsurg.2008.12.018.
Mandarano M, Bellezza G, Belladonna ML, et al. Indoleamine 2,3-Dioxygenase 2 Immunohistochemical expression in resected human non-small cell lung cancer: a potential new prognostic tool. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.00839.
Liu Y, Xu P, Liu H, et al. Silencing IDO2 in dendritic cells: a novel strategy to strengthen cancer immunotherapy in a murine lung cancer model. Int J Oncol. 2020;57:587–97. https://doi.org/10.3892/ijo.2020.5073.c.
Napolioni V, Pariano M, Borghi M, et al. Genetic polymorphisms affecting IDO1 or IDO2 activity differently associate with aspergillosis in humans. Front Immunol. 2019;10:890. https://doi.org/10.3389/fimmu.2019.00890.
Liu Y, Zhang Y, Zheng X, et al. Gene silencing of indoleamine 2, 3-dioxygenase 2 in melanoma cells induces apoptosis through the suppression of NAD+ and inhibits in vivo tumor growth. Oncotarget. 2016. https://doi.org/10.18632/oncota.8617.
Metz R, Smith C, DuHadaway JB, et al. Mandik-Nayak, IDO2 is critical for IDO1-mediated T cell regulation and exerts a non-redundant function in inflammation. Int Immunol. 2014;26:357–67. https://doi.org/10.1093/intimm/dxz003.
Van-Baren N, Van den Eynde BJ. Tryptophan-degrading enzymes in tumoral immune resistance. Front Immunol. 2015;6:34. https://doi.org/10.3389/fimmu.2015.00034.
Opitz CA, Litzenburger UM, Sahm F, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203.
Pilotte L, Larrieu P, Stroobant V, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA. 2012;109:2497–502. https://doi.org/10.1073/pnas.1113873109.
Dolusic E, Larrieu P, Moineaux L, et al. Tryptophan 2, 3-dioxygenase (TDO) inhibitors 3-(2-(pyridyl) ethenyl) indoles as potential anticancer immunomodulators. J Med Chem. 2011. https://doi.org/10.1021/jm2006782.
Hoffmann D, Dvorakova T, Stroobant V, et al. Tryptophan 2,3-dioxygenase expression identified in human hepatocellular carcinoma cells and in intratumoral pericytes of most cancers. Cancer Immunol Res. 2020;8:19–31. https://doi.org/10.1158/2326-6066.cir-19-0040.
Li T, Wang S, Li Z, et al. TDO2 promotes the EMT of hepatocellular carcinoma through Kyn-AhR Pathway. Front Oncol. 2021;10:3008. https://doi.org/10.3389/fonc.2020.562823.
Yu C, Rao D, Zhu H, et al. TDO2 was downregulated in hepatocellular carcinoma and inhibited cell proliferation by upregulating the expression of p21 and p27. BioMed Res Int. 2021. https://doi.org/10.1155/2021/4708439.
Du L, Xing Z, Tao B, et al. Both IDO1 and TDO contribute to the malignancy of gliomas via the Kyn–AhR–AQP4 signaling pathway. Signal Transduct Target Ther. 2020;5:1–13. https://doi.org/10.1038/s41392-019-0103-4.
Liang H, Li T, Fang X, et al. IDO1/TDO dual inhibitor RY103 targets Kyn-AhR pathway and exhibits preclinical efficacy on pancreatic cancer. Cancer Lett. 2021;522:32–43. https://doi.org/10.1016/j.canlet.2021.09.012.
Reed MR, Maddukuri L, Ketkar A, et al. Inhibition of tryptophan 2, 3-dioxygenase impairs DNA damage tolerance and repair in glioma cells. NAR cancer. 2021. https://doi.org/10.1093/narcan/zcab014.
Hsu HL, Hung JY, Chiang SY, et al. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget. 2016. https://doi.org/10.8632/oncotarget.8488.
Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–61. https://doi.org/10.4049/jimmunol.176.11.6752.
Wu Z, Yan L, Lin J, Ke K, Yang W. Constitutive TDO2 expression promotes liver cancer progression by an autocrine IL-6 signaling pathway. Cancer Cell Int. 2021;21:1–9. https://doi.org/10.1186/s12935-021-02228-9.
Schramme F, Crosignani S, Frederix K, et al. Inhibition of tryptophan-dioxygenase activity increases the antitumor efficacy of immune checkpoint inhibitors. Cancer Immunol Res. 2020;8:32–45. https://doi.org/10.1158/2326-6066.cir-19-0041.
Li S, Li S, Zhao Y, Zhang B, et al. A comprehensive analysis of TDO2 expression in immune cells and characterization of immune cell phenotype in TDO2 knockout mice. Transgenic Res. 2021;30:781–97. https://doi.org/10.1007/s11248-021-00281-8.
Gullapalli S, Roychowdhury A, Khaladkar T, et al. EPL-1410, a novel fused heterocycle based orally active dual inhibitor of IDO1/TDO2, as a potential immune-oncology therapeutic. Can Res. 2018. https://doi.org/10.1158/1538-7445.AM2018-1701.
Kim C, Lee NK, Kim JS, et al. An oral dual inhibitor of IDO and TDO enhances anti-cancer immunity and synergizes with immune checkpoint blockade. Annal Oncol. 2018. https://doi.org/10.1093/annonc/mdy288.039.
Naing A, Eder JP, Piha-Paul SA, et al. Preclinical investigations and a first-in-human phase I trial of M4112, the first dual inhibitor of indoleamine 2, 3-dioxygenase 1 and tryptophan 2, 3-dioxygenase 2, in patients with advanced solid tumors. J Immunother Cancer. 2020. https://doi.org/10.1136/jitc-2020-000870.
Walther DJ, Peter JU, Bashammakh S, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299:76–76. https://doi.org/10.1126/science.1078197.
Platten M, Nollen EAA, Rohrig UF, et al. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat Rev Drug Discov. 2019;18:379–401. https://doi.org/10.1038/s41573-019-0016-5.
O’Connell PJ, Wang X, Leon-Ponte M, et al. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood. 2006;107:1010–7. https://doi.org/10.1182/blood-2005-07-2903.
de las Casas-Engel M, Domínguez-Soto A, Sierra-Filardi E, et al. (2013) Serotonin skews human macrophage polarization through HTR2B and HTR7. J Immunol. https://doi.org/10.4049/jimmunol.1201133.
Nieto C, Rayo I, de Las C-E, et al. Serotonin (5-HT) Shapes the macrophage gene profile through the 5-ht2b–dependent activation of the aryl hydrocarbon receptor. J Immunol. 2020;204:2808–17. https://doi.org/10.4049/jimmunol.1901531.
Gwynne WD, Hallett RM, Girgis-Gabardo A, et al. Serotonergic system antagonists target breast tumor initiating cells and synergize with chemotherapy to shrink human breast tumor xenografts. Oncotarget. 2017. https://doi.org/10.8632/oncotarget.16646.
Nocito A, Dahm FW, Jochum JH, et al. Serotonin regulates macrophage-mediated angiogenesis in a mouse model of colon cancer allografts. Cancer Res. 2008;68:5152–8. https://doi.org/10.1158/0008-5472.can-08-0202.
Soll C, Jang JH, Riener MO, et al. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology. 2010;51:1244–54. https://doi.org/10.1002/hep.23441.
Ge C, Yan J, Yuan X, Xu G. A positive feedback loop between tryptophan hydroxylase 1 and β-catenin/ZBP-89 signalling promotes prostate cancer progression. Front Oncol. 2022. https://doi.org/10.3389/fonc.2022.923307.
Nowak EC, de Vries VC, Wasiuk A, et al. Tryptophan hydroxylase-1 regulates immune tolerance and inflammation. J Exp Med. 2012;209:2127. https://doi.org/10.1084/jem.20120408.
Hagiwara A, Nakamura Y, Nishimoto R, et al. Induction of tryptophan hydroxylase in the liver of sc tumor model of prostate cancer. Cancer Sci. 2020;111:1218–27. https://doi.org/10.1111/cas.14333.
Matthes S, Bader M. Peripheral serotonin synthesis as a new drug target. Trends Pharmacol Sci. 2018;39:560–72. https://doi.org/10.1016/j.tips.2018.03.004.
Patil MD, Bhaumik J, Babykutty S, et al. Arginine dependence of tumor cells: targeting a chink in cancer’s armor. Oncogene. 2016;35:4957–72. https://doi.org/10.1038/onc.2016.37.
Husson A, Brasse-Lagnel C, Fairand A, et al. Argininosuccinate synthetase from the urea cycle to the citrulline-NO cycle. Eur J Biochem. 2003;270:1887–99. https://doi.org/10.1046/j.1432-1033.2003.03559.x.
Coulter JA, McCarthy HO, Xiang J, et al. Nitric oxide-a novel therapeutic for cancer. Nitric Oxide. 2008;19:192–8. https://doi.org/10.1016/j.niox.2008.04.023.
Menjivar RE, Halbrook C, Velez A, et al. (2019) Abstract A31: investigating the effect of myeloid arg1 deletion on tumor growth and CD8+ T cell infiltration and activation in pancreatic cancer. Cancer Res
Feun LG, Marini A, Walker G, et al. Negative argininosuccinate synthetase expression in melanoma tumours may predict clinical benefit from arginine-depleting therapy with pegylated arginine deiminase. Br J cancer. 2012;106:1481–5. https://doi.org/10.1038/bjc.2012.106.
Yang JS, Wang CC, Qiu JD, et al. Arginine metabolism: a potential target in pancreatic cancer therapy. Chin Med J. 2021;134:28–37. https://doi.org/10.1097/CM9.0000000000001216.
Delage B, Fennell DA, Nicholson L, et al. Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer. 2012;126:2762–72. https://doi.org/10.1002/ijc.25202.
Rodriguez PC, Zea AH, DeSalvo J, et al. l-arginine consumption by macrophages modulates the expression of CD3ζ chain in T lymphocytes. J Immunol. 2003;171:1232–9. https://doi.org/10.4049/jimmunol.171.3.1232.
Zea AH, Rodriguez PC, Atkins MB, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005. https://doi.org/10.1158/0008-5472.CAN-04-4505.
Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T lymphocyte cell-cycle progression. Blood. 2007;109:1568–73. https://doi.org/10.1182/blood-2006-06-031856.
Riess C, Shokraie F, Classen CF, et al. Arginine-depleting enzymes–an increasingly recognized treatment strategy for therapy-refractory malignancies. Cell Physiol Biochem. 2018;51(2):854–70. https://doi.org/10.1159/000495382.
Giatromanolaki A, Harris AL, Koukourakis MI. The prognostic and therapeutic implications of distinct patterns of argininosuccinate synthase 1 (ASS1) and arginase-2 (ARG2) expression by cancer cells and tumor stroma in non-small-cell lung cancer. Cancer Metab. 2021;9:28. https://doi.org/10.1186/s40170-021-00264-7.
Grzywa TM, Sosnowska A, Matryba P, Rydzynska Z, Jasinski M, Nowis D, Golab J. Myeloid cell-derived arginase in cancer immune response. Front Immunol. 2020;11:938. https://doi.org/10.3389/fimmu.2020.00938.
Munder M, Schneider H, Luckner C, et al. Suppression of T cell functions by human granulocyte arginase. Blood. 2006;108:1627–34. https://doi.org/10.1182/blood-2006-11-010389.
de Coaña YP, Poschke I, Gentilcore G, et al. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their arginase1 production cancer immunol. Res. 2013;3:158–62. https://doi.org/10.1158/2326-6066.CIR-13-0016.
Steggerda SM, Bennett MK, Chen J, et al. Murray, Inhibition of arginase by CB-1158 blocks myeloid cell-mediated immune suppression in the tumor microenvironment. J Immunother Cancer. 2017;5:101. https://doi.org/10.1186/s40425-017-0308-4.
Zhang H, Li ZL, Ye SB, et al. Myeloid-derived suppressor cells inhibit T cell proliferation in human extranodal NK/T cell lymphoma: a novel prognostic indicator. Cancer Immunol Immunother. 2015;64:1587–99. https://doi.org/10.1007/s00262-015-1765-6.
Molon B, Ugel S, Del Pozzo F, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–62. https://doi.org/10.1084/jem.20101956.
Zea AH, Rodriguez PC, Culotta KS, et al. L-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell Immunol. 2004;232:21–31. https://doi.org/10.1016/j.cellimm.2005.01.004.
Norian LA, Rodriguez PC, O’Mara LA, et al. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res. 2009;69:3086–94. https://doi.org/10.1158/0008-5472.CAN-08-2826.
Modolell M, Choi BS, Ryan RO, et al. Local suppression of T cell responses by arginase-induced L-arginine depletion in nonhealing leishmaniasis. PLoS Negl Trop Dis. 2009;3: e480. https://doi.org/10.1371/journal.pntd.0000480.
Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–94. https://doi.org/10.4049/jimmunol.176.4.2085.
Izzo F, Marra P, Beneduce G, et al. Pegylated arginine deiminase treatment of patients with unresectable hepatocellular carcinoma: results from phase I/II studies. J Clin Oncol. 2004;22:1815–22. https://doi.org/10.1200/JCO.2004.11.120.
Tsai HJ, Jiang SS, Hung WC, et al. A phase II study of arginine deiminase (ADI-PEG20) in relapsed/refractory or poor-risk acute myeloid leukemia patients. Sci rep. 2017;7:1–10. https://doi.org/10.1038/s41598-017-10542-4.
Lowery MA, Yu KH, Kelsen DP, et al. A phase 1/1B trial of ADI-PEG 20 plus nab-paclitaxel and gemcitabine in patients with advanced pancreatic adenocarcinoma. Cancer. 2017. https://doi.org/10.1002/cncr.30897.
Yao S, Janku F, Subbiah V, et al. Phase 1 trial of ADI-PEG20 plus cisplatin in patients with pretreated metastatic melanoma or other advanced solid malignancies. Br J Cancer. 2021;124:1533–9. https://doi.org/10.1038/s41416-020-01230-8.
Tsai HJ, Hsiao HH, Hsu YT, et al. Phase I study of ADI-PEG20 plus low-dose cytarabine for the treatment of acute myeloid leukemia. Cancer med. 2021;10:2946–55. https://doi.org/10.1002/cam4.3871.
Ascierto PA, Scala S, Castello G, et al. Pegylated arginine deiminase treatment of patients with metastatic melanoma: results from phase I and II studies. J Clin Oncol. 2005;23:7660–8. https://doi.org/10.1200/JCO.2005.02.0933.
Ott PA, Carvajal RD, Pandit-Taskar N, et al. Phase I/II study of pegylated arginine deiminase (ADI-PEG 20) in patients with advanced melanoma. Investig New Drugs. 2013;31:425–34. https://doi.org/10.1038/bjc.2011.524.
Yau T, Cheng PN, Chan P, et al. Preliminary efficacy, safety, pharmacokinetics, pharmacodynamics and quality of life study of pegylated recombinant human arginase 1 in patients with advanced hepatocellular carcinoma. Investig New Drugs. 2015;33:496–504. https://doi.org/10.1007/s10637-014-0200-8.
Yau T, Cheng PN, Chan P, et al. A phase 1 dose-escalating study of pegylated recombinant human arginase 1 (Peg-rhArg1) in patients with advanced hepatocellular carcinoma. Invest new drugs. 2013;31:99–107. https://doi.org/10.1007/s10637-012-9807-9.
Brin E, Wu K, Lu HT, et al. PEGylated arginine deiminase can modulate tumor immune microenvironment by affecting immune checkpoint expression, decreasing regulatory T cell accumulation and inducing tumor T cell infiltration. Oncotarget. 2017. https://doi.org/10.1632/oncotarget.19564.
Chen CL, Hsu SC, Ann DK, et al. Arginine signaling and cancer metabolism. Cancers. 2021;13:3541. https://doi.org/10.3390/cancers13143541.
Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. https://doi.org/10.1042/0264-6021:3570593.
Hu Y, Xiang J, Su L, et al. The regulation of nitric oxide in tumor progression and therapy. J Int Med Res. 2020. https://doi.org/10.1177/0300060520905985.
Jayaraman P, Parikh F, Lopez-Rivera E, et al. Tumor-expressed iNOS controls induction of functional myeloid derived suppressor cells (MDSC) through modulation of VEGF release. J Immunol. 2012;188:5365. https://doi.org/10.4049/jimmunol.1103553.
Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–34. https://doi.org/10.1182/blood-2006-02-002246.
Lum HD, Schmidt BIN, BE, et al. Tumoristatic effects of anti-CD40 mAb-activated macrophages involve nitric oxide and tumour necrosis factor-alpha. Immunology. 2006. https://doi.org/10.1111/j.1365-2567.2006.02366.x.
Douguet L, Bod L, Lengagne R, et al. Nitric oxide synthase 2 is involved in the pro-tumorigenic potential of γδ17 T cells in melanoma. OncoImmunology. 2016;5: e1208878. https://doi.org/10.1080/2162402X.2016.1208878.
Ding Z, Ogata D, Roszik J, et al. iNOS associates with poor survival in melanoma: a role for nitric oxide in the PI3K-AKT pathway stimulation and PTEN S-nitrosylation. Front Oncol. 2021;11: 631766. https://doi.org/10.3389/fonc.2021.631766.
Markowitz J, Wang J, Vangundy Z, et al. Nitric oxide mediated inhibition of antigen presentation from DCs to CD4+ T cells in cancer and measurement of STAT1 nitration. Sci Rep. 2017;7:1–13. https://doi.org/10.1038/s41598-017-14970-0.
Ito H, Ando T, Seishima M. Inhibition of iNOS activity enhances the antitumor effects of alpha-galactosylceramide in established murine cancer model. Oncotarget. 2015. https://doi.org/10.8632/oncotarget.6172.
Lahdenranta J, Hagendoorn J, Padera TP, et al. Endothelial nitric oxide synthase mediates lymphangiogenesis and lymphatic metastasis. Cancer Res. 2009;69:2801–8. https://doi.org/10.1158/0008-5472.CAN-08-4051.
Barcińska E, Wierzbicka J, Zauszkiewicz-Pawlak A, et al. Role of oxidative and nitro-oxidative damage in silver nanoparticles cytotoxic effect against human pancreatic ductal adenocarcinoma cells. Oxid Med Cell Longev. 2018. https://doi.org/10.1155/2018/8251961.
Chen J, Wang T, Xu S, et al. Discovery of novel antitumor nitric oxide-donating β-elemene hybrids through inhibiting the PI3K/Akt pathway. Eur J Med Chem. 2017;135:414–23. https://doi.org/10.1016/j.ejmech.2017.04.045.
Gonçalves DA, Xisto R, Gonçalves JD, et al. Imbalance between nitric oxide and superoxide anion induced by uncoupled nitric oxide synthase contributes to human melanoma development. Int J Biochem Cell Biol. 2019;115: 105592. https://doi.org/10.1016/j.biocel.2019.105592.
Salaroglio IC, Gazzano E, Abdullrahman A, et al. Increasing intratumor C/EBP-β LIP and nitric oxide levels overcome resistance to doxorubicin in triple negative breast cancer. J Exp Clin Cancer Res. 2018;37:286. https://doi.org/10.1186/s13046-018-0967-0.
Allard B, Longhi MS, Robson SC, et al. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev. 2017;276:121–44. https://doi.org/10.1111/imr.12528.
Ghiringhelli F, Bruchard M, Chalmin F, et al. Production of adenosine by ectonucleotidases: a key factor in tumor immunoescape. J Biomed Biotechnol. 2012. https://doi.org/10.1155/2012/473712.
Beavis PA, Stagg J, Darcy PK, et al. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 2012;33:231–7. https://doi.org/10.1016/j.it.2012.02.009.
Morello S, Capone M, Sorrentino C, et al. Soluble CD73 as biomarker in patients with metastatic melanoma patients treated with nivolumab. J Transl Med. 2017;15:1–9. https://doi.org/10.1186/s12967-017-1348-8.
Longhi MS, Robson SC, Bernstein SH, et al. Biological functions of ecto-enzymes in regulating extracellular adenosine levels in neoplastic and inflammatory disease states. J Mol Med. 2013;91:165–72. https://doi.org/10.1007/s00109-012-0991-z.
Kumar M, Lowery R, Kumar V. High-throughput screening assays for cancer immunotherapy targets: ectonucleotidases CD39 and CD73. SLAS Discov. 2020;25:320–6. https://doi.org/10.1177/2472555219893632.
Bastid J, Cottalorda-Regairaz A, Alberici G, et al. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene. 2013;32:1743–51. https://doi.org/10.1038/onc.2012.269.
Antonioli L, Pacher P, Vizi ES, et al. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–67. https://doi.org/10.1016/j.molmed.2013.03.005.
Wink MR, Tamajusuku AS, Braganhol E, et al. Thyroid hormone upregulates ecto-5’-nucleotidase/CD73 in C6 rat glioma cells. Mol Cell Endocrinol. 2003;205:107–14. https://doi.org/10.1016/s0303-7207(03)00197-7.
Mandapathil M, Szczepanski MJ, Szajnik M, et al. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin cancer res. 2009;15:6348–57. https://doi.org/10.1158/1078-0432.ccr-09-1143.
Theodoraki MN, Hoffmann TK, Jackson EK, et al. Exosomes in HNSCC plasma as surrogate markers of tumour progression and immune competence. Clin Exp Immunol. 2018;194:67–78. https://doi.org/10.1111/cei.13157.
Sadej R, Spychala J, Skladanowski AC. Expression of ecto-5’-nucleotidase (eN, CD73) in cell lines from various stages of human melanoma. Melanoma Res. 2006;16:213–22. https://doi.org/10.1097/01.cmr.0000215030.69823.11.
Dzhandzhugazyan KN, Kirkin AF, Thor Straten P, et al. Ecto-ATP diphosphohydrolase/CD39 is overexpressed in differentiated human melanomas. FEBS Lett. 1990. https://doi.org/10.1016/s0014-5793(98)00603-6.
Retseck J, Nasr A, Lin Y, et al. Long term impact of CTLA4 blockade immunotherapy on regulatory and effector immune responses in patients with melanoma. J Transl Med. 2018;16:184. https://doi.org/10.1186/s12967-018-1563-y.
Kondo T, Nakazawa T, Murata SI, et al. Expression of CD73 and its ecto-5′-nucleotidase activity are elevated in papillary thyroid carcinomas. Histopathology. 2006;48:612–4. https://doi.org/10.1111/j.1365-2559.2005.02277.x.
Zhou X, Zhi X, Zhou P, et al. Effects of ecto-5’-nucleotidase on human breast cancer cell growth in vitro and in vivo. Oncol Rep. 2007;17:1341–6. https://doi.org/10.3892/or.17.6.1341.
Buffon A, Wink MR, Ribeiro BV, et al. (2007) NTPDase and 5’ ecto-nucleotidase expression profiles and the pattern of extracellular ATP metabolism in the the Walker 256 tumor Biochimica et Biophysica Acta (BBA)-General Subjects https://doi.org/10.1016/j.bbagen.2007.05.004
Zhi X, Wang Y, Yu J, et al. Potential prognostic biomarker CD73 regulates epidermal growth factor receptor expression in human breast cancer. IUBMB Life. 2012;64:911–20. https://doi.org/10.1002/iub.1086.
Künzli BM, Berberat PO, Giese T, et al. Upregulation of CD39/NTPDases and P2 receptors in human pancreatic disease. Am J Physiol Gastrointest Liver Phys. 2007;292:G223–30. https://doi.org/10.1152/ajpgi.00259.2006.
Sun X, Wu Y, Gao W, et al. CD39/ENTPD1 expression by CD4+ Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterol. 2006;139:1030–40. https://doi.org/10.1053/j.gastro.2010.05.007.
Wu XR, He XS, Chen YF, et al. High expression of CD73 as a poor prognostic biomarker in human colorectal cancer. J Surg Oncol. 2012;106:130–7. https://doi.org/10.1002/jso.23056.
Künzli BM, Bernlochner MI, Rath S, et al. Impact of CD39 and purinergic signalling on the growth and metastasis of colorectal cancer. Purinergic Signal. 2011;2:231–41. https://doi.org/10.1007/s11302-011-9228-9.
Stella J, Bavaresco L, Braganhol E, et al. Differential ectonucleotidase expression in human bladder cancer cell lines. Urol Oncol. 2012;28:260–7. https://doi.org/10.1016/j.urolonc.2009.01.035.
Häusler SF, Montalbán del Barrio I, Strohschein J, et al. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother. 2011. https://doi.org/10.1007/s00262-011-1040-4.
Montalbán del Barrio I, Penski C, Schlahsa L, et al. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages–a self-amplifying, CD39-and CD73-dependent mechanism for tumor immune escape. J Immunother Cancer. 2016. https://doi.org/10.1186/s40425-016-0154-9.
Salimu J, Webber J, Gurney M, et al. Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J Extracell Vesicles. 2017;6:1368823. https://doi.org/10.1080/20013078.2017.1368823.
Stagg J, Beavis PA, Divisekera U, et al. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 2012;72:2190–6. https://doi.org/10.1158/0008-5472.CAN-12-0420.
Giatromanolaki A, Kouroupi M, Pouliliou S, et al. Ectonucleotidase CD73 and CD39 expression in non-small cell lung cancer relates to hypoxia and immunosuppressive pathways. Life Sci. 2020;259: 118389. https://doi.org/10.1016/j.lfs.2020.118389.
Morandi F, Marimpietri D, Horenstein AL, et al. Microvesicles expressing adenosinergic ectoenzymes and their potential role in modulating bone marrow infiltration by neuroblastoma cells. Oncoimmunol. 2019. https://doi.org/10.1080/2162402X.2019.1574198.
Pulte D, Olson KE, Broekman MJ, et al. CD39 activity correlates with stage and inhibits platelet reactivity in chronic lymphocytic leukemia. J Trans Med. 2007;5:1–10. https://doi.org/10.1186/1479-5876-5-23.
Perry C, Hazan-Halevy I, Kay S, et al. Increased CD39 expression on CD4+ T lymphocytes has clinical and prognostic significance in chronic lymphocytic leukemia. Ann Hematol. 2012;91:1271–9. https://doi.org/10.1007/s00277-012-1425-2.
Cai Y, Feng L, Yuan D, et al. The role of CD39/CD73/Ado/A2AR axis and HIF-1α in chronic lymphocytic leukemia. Blood. 2018;132:4406.
Aroua N, Boet E, Ghisi M, et al. Extracellular ATP and CD39 activate cAMP-mediated mitochondrial stress response to promote cytarabine resistance in acute myeloid leukemia. Cancer Discov. 2020;10:1544–65. https://doi.org/10.1158/2159-8290.CD-19-1008.
Hilchey SP, Kobie JJ, Cochran MR, et al. Human follicular lymphoma CD39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J Immunol. 2009;183:6157–66. https://doi.org/10.4049/jimmunol.0900475.
Clayton A, Al-Taei S, Webber J, et al. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011. https://doi.org/10.4049/jimmunol.1003884.
Yang R, Elsaadi S, Misund K, et al. Conversion of ATP to adenosine by CD39 and CD73 in multiple myeloma can be successfully targeted together with adenosine receptor A2A blockade. J Immunother Cancer. 2020. https://doi.org/10.1136/jitc-2020-000610.
Emens L, Powderly J, Fong L, et al. (2017) CPI-444, an oral adenosine A2a receptor (A2aR) antagonist, demonstrates clinical activity in patients with advanced solid tumors. Cancer Res
Moesta AK, Li XY, Smyth MJ. Targeting CD39 in cancer. Nat Rev Immunol. 2020;20:739–55. https://doi.org/10.1038/s41577-020-0376-4.
Perrot I, Michaud HA, Giraudon-Paoli M, et al. Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 2019;27:2411–25. https://doi.org/10.1016/j.celrep.2019.04.091.
Li XY, Moesta AK, Xiao C, et al. Targeting CD39 in cancer reveals an extracellular ATP-and inflammasome-driven tumor immunity. Cancer Discov. 2019;9:1754–73. https://doi.org/10.1158/2159-8290.CD-19-0541.
Matissek S, Sicheva MP, Koseoglu S, et al. The fully human antibody SRF617 is a potent enzymatic inhibitor of CD39 with strong immunomodulatory activity (Poster 652). J Immunother Cancer. 2021;7:283.
Eiger D, Maurer C, Brandao M, et al. First findings from SYNERGY, a phase I/II trial testing the addition of the anti-CD73 oleclumab (O) to the anti-PD-L1 durvalumab (D) and chemotherapy (ChT) as first line therapy for patients (pts) with metastatic triple-negative breast cancer (mTNBC). Ann Oncol. 2020;31:S386–7. https://doi.org/10.1016/j.annonc.2020.09.004.
Bendell JC, LoRusso P, Overman MJ, et al. Safety and efficacy of the anti-CD73 monoclonal antibody (mAb) oleclumab±durvalumab in patients (pts) with advanced colorectal cancer (CRC), pancreatic ductal adenocarcinoma (PDAC), or EGFR-mutant non-small cell lung cancer (EGFRm NSCLC). J Clin Oncol. 2021;39:9047–9047. https://doi.org/10.1200/JCO.2021.39.15_suppl.9047.
Powderly J, Bendell JC, Carneiro BA, et al. 1073TiP A phase I, first-in-human, multicenter, open-label, dose-escalation study of IPH5201 as monotherapy or in combination with durvalumab±oleclumab in advanced solid tumours. Ann Oncol. 2020;31:S728–9. https://doi.org/10.1016/j.annonc.2020.08.1193.
Petruk N, Tuominen S, Åkerfelt M, Mattsson J, Sandholm J, Nees M, Yegutkin GG, Jukkola A, Tuomela J, Selander KS. CD73 facilitates EMT progression and promotes lung metastases in triple-negative breast cancer. Sci rep. 2021;11:1–3. https://doi.org/10.1038/s41598-021-85379-z.
Ma XL, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis. J Hematol Oncol. 2019;12:37. https://doi.org/10.1186/s13045-019-0724-7.
Allard B, Turcotte M, Spring K, Pommey S, et al. Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer. 2014;134:1466–73. https://doi.org/10.1002/ijc.28456.
Zhou L, Jia S, Chen Y, et al. The distinct role of CD73 in the progression of pancreatic cancer. J Mol Med. 2019;97:803–15. https://doi.org/10.1007/s00109-018-01742-0.
Turiello R, Capone M, Giannarelli D, et al. Serum CD73 is a prognostic factor in patients with metastatic melanoma and is associated with response to anti-PD-1 therapy. J Immunother Cancer. 2020. https://doi.org/10.1136/jitc-2020-001689.
Schäkel L, Mirza S, Winzer R, et al. Protein kinase inhibitor ceritinib blocks ectonucleotidase CD39-a promising target for cancer immunotherapy. J ImmunoTher Cancer. 2022. https://doi.org/10.1136/jitc-2022-004660.
Schäkel L, Schmies CC, Idris RM, Luo X, Lee SY, Lopez V, Mirza S, Vu TH, Pelletier J, Sevigny J, Namasivayam V. Nucleotide analog ARL67156 as a lead structure for the development of CD39 and dual CD39/CD73 ectonucleotidase inhibitors. Front Pharmacol. 2020. https://doi.org/10.3389/fphar.2020.01294.
Bastid J, Regairaz A, Bonnefoy N, et al. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer immunol res. 2015;3:254–65. https://doi.org/10.1158/2326-6066.CIR-14-0018.
Oiseth SJ, Aziz MS. (2017) Cancer immunotherapy: a brief review of the history, possibilities, and challenges ahead. J. Cancer Metastasis Treat https://doi.org/10.20517/2394-4722.2017.41
Günther J, Däbritz J, Wirthgen E. Limitations and off-target effects of tryptophan-related IDO inhibitors in cancer treatment. Front Immunol. 2019;10:1801. https://doi.org/10.3389/fimmu.2019.01801.
Van den Eynde BJ, van Baren N, Baurain JF. Is there a clinical future for IDO1 inhibitors after the failure of epacadostat in melanoma? Annual Rev Cancer Bio. 2020;4:241–56. https://doi.org/10.1146/annurev-cancerbio-030419-033635.
Battastini AM, Figueiró F, Leal DB, et al. CD39 and CD73 as promising therapeutic targets: what could be the limitations? Front Pharmacol. 2021. https://doi.org/10.3389/fphar.2021.633603.
Acknowledgements
Authors acknowledge the support from Maharishi Markandeshwar (Deemed to be University), Mullana, Ambala, Haryana, India.
Funding
The authors are grateful to Maharishi Markandeshwar (Deemed to be University) for providing financial support for writing this review article.
Author information
Authors and Affiliations
Contributions
Study concept and design: RVS and AKS. Data acquisition: GC, RK, RVS, and AKS. Analysis and interpretation of data: RVS, AKS, and AKS. Drafting the manuscript: GC and RK. Critical revision of the manuscript: SC, AM, and AKS. Study supervision: RVS. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandan, G., Saini, A.K., Kumari, R. et al. The exploitation of enzyme-based cancer immunotherapy. Human Cell 36, 98–120 (2023). https://doi.org/10.1007/s13577-022-00821-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00821-2