Abstract
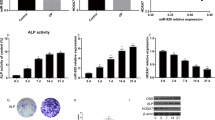
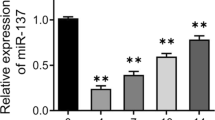
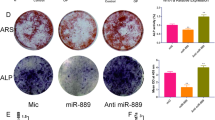
Osteoporosis is a disorder characterized by reduced bone mass, disruption of bone microarchitecture, and a propensity to fracture. The osteogenic differentiation of human bone mesenchymal stromal cells (hBMSCs) exerts a critical effect on preventing bone loss during osteoporosis. Herein, the study recognized miR-100-5p as a deregulated miRNA during osteoporosis (upregulated) and BMSC osteogenic differentiation (downregulated). miR-100-5p was upregulated in osteoporosis patients-isolated BMSCs compared to non-osteoporosis trauma patients-isolated BMSCs. hBMSCs, overexpression inhibited hBMSC proliferation and osteogenic differentiation, whereas miR-100-5p inhibition exerted opposite effects. TMEM135 was downregulated in osteoporosis and upregulated in differentiated osteoblasts, as well as downregulated upon the overexpression of miR-100-5p. MiR-100-5p directly targeted and inhibited TMEM135. In hBMSCs, TMEM135 silencing also inhibited hBMSC osteogenic differentiation. When co-transfected to hBMSCs, antagomir-100-5p promoted, whereas TMEM135 silencing inhibited hBMSC osteogenic differentiation; TMEM135 knockdown dramatically attenuated the effects of miR-100-5p inhibition. Taken together, miR-100-5p forms a regulatory axis with TMEM135 by direct binding. The miR-100-5p/TMEM135 axis modulates hBMSC differentiation into osteoblast. Considering the critical effect of BMSC osteogenesis on osteoporosis, this axis might play a role in osteoporosis, and further in vivo and clinical investigations are required.





Similar content being viewed by others
Availability of data and materials
All the data and materials in the manuscript is available.
References
Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276–87.
Hayashi M, et al. Autoregulation of osteocyte Sema3A orchestrates estrogen action and counteracts bone aging. Cell Metab. 2019;29(3):627–37.
Berendsen AD, Olsen BR. Osteoblast-adipocyte lineage plasticity in tissue development, maintenance and pathology. Cell Mol Life Sci. 2014;71(3):493–7.
Mourelatos Z. Small RNAs: the seeds of silence. Nature. 2008;455(7209):44–5.
Schreck C, et al. Niche WNT5A regulates the actin cytoskeleton during regeneration of hematopoietic stem cells. J Exp Med. 2017;214(1):165–81.
Li XH, et al. Integrated analysis of differential miRNA and mRNA expression profiles in human radioresistant and radiosensitive nasopharyngeal carcinoma cells. PLoS ONE. 2014;9(1): e87767.
Li CJ, et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J Clin Invest. 2015;125(4):1509–22.
Huang J, et al. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28(2):357–64.
Liu S, et al. MSC transplantation improves osteopenia via epigenetic regulation of notch signaling in lupus. Cell Metab. 2015;22(4):606–18.
Tang Y, et al. MicroRNA-99a is a novel regulator of KDM6B-mediated osteogenic differentiation of BMSCs. J Cell Mol Med. 2018;22(4):2162–76.
Li Y, et al. miR-149-3p Regulates the switch between adipogenic and osteogenic differentiation of BMSCs by targeting FTO. Mol Ther Nucleic Acids. 2019;17:590–600.
Rotondo Dottore G, et al. Genetic profiling of orbital fibroblasts from patients with graves’ orbitopathy. J Clin Endocrinol Metab. 2021;106(5):e2176–90.
Rio DC, et al. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harbor Protoc. 2010;2010(6):pdb.prot5439.
Ha M, et al. Interspecies regulation of microRNAs and their targets. Biochim Biophys Acta. 2008;1779(11):735–42.
Scheideler M, et al. Comparative transcriptomics of human multipotent stem cells during adipogenesis and osteoblastogenesis. BMC Genomics. 2008;9:340.
Li T, et al. MicroRNA expression profile of dexamethasone-induced human bone marrow-derived mesenchymal stem cells during osteogenic differentiation. J Cell Biochem. 2014;115(10):1683–91.
Gu H, et al. Conditions inducing excessive O-GlcNAcylation inhibit BMP2-induced osteogenic differentiation of C2C12 cells. Int J Mol Sci. 2018;19(1):202.
Lu SY, et al. The osteogenesis-promoting effects of alpha-lipoic acid against glucocorticoid-induced osteoporosis through the NOX4, NF-kappaB, JNK and PI3K/AKT pathways. Sci Rep. 2017;7(1):3331.
Wu SM, et al. Estrogen enhances activity of Wnt signaling during osteogenesis by inducing Fhl1 expression. J Cell Biochem. 2015;116(7):1419–30.
Wang Z, et al. A regulatory loop containing miR-26a, GSK3beta and C/EBPalpha regulates the osteogenesis of human adipose-derived mesenchymal stem cells. Sci Rep. 2015;5:15280.
Gruber HE, et al. Human annulus progenitor cells: analyses of this viable endogenous cell population. J Orthop Res. 2016;34(8):1351–60.
Ferrari SL. Prevention of fractures in patients with osteoporosis. Lancet. 2018;391(10117):184–6.
Lian JB, et al. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8(4):212–27.
Liu Y, et al. Epigenetic mechanisms of bone regeneration and homeostasis. Prog Biophys Mol Biol. 2016;122(2):85–92.
Wei B, et al. Differentially expressed microRNAs in conservatively treated nontraumatic osteonecrosis compared with healthy controls. Biomed Res Int. 2018;2018:9015758.
Kelch S, et al. miRNAs in bone tissue correlate to bone mineral density and circulating miRNAs are gender independent in osteoporotic patients. Sci Rep. 2017;7(1):15861.
Wang R, et al. Searching for valuable differentially expressed miRNAs in postmenopausal osteoporosis by RNA sequencing. J Obstet Gynaecol Res. 2020;46(7):1183–92.
Frith JE, et al. Mechanically-sensitive miRNAs bias human mesenchymal stem cell fate via mTOR signalling. Nat Commun. 2018;9(1):257.
Zeng Y, et al. MicroRNA-100 regulates osteogenic differentiation of human adipose-derived mesenchymal stem cells by targeting BMPR2. FEBS Lett. 2012;586(16):2375–81.
Dai Z, Wei G. Inhibition of miRNA-100 facilitates bone regeneration defects of mesenchymal stem cells in osteoporotic mice through the protein kinase B pathway. Bioengineered. 2022;13(1):963–73.
Yang W, et al. Exosomal miR-100-5p inhibits osteogenesis of hBMSCs and angiogenesis of HUVECs by suppressing the BMPR2/Smad1/5/9 signalling pathway. Stem Cell Res Ther. 2021;12(1):390.
Acknowledgements
This study was supported by Special Precision Medicine Project of the National Key R&D Program from the Science and Technology Ministry of China (2016YFC0901203) and the Cultivate project 2021 for National Natural Science Foundation of China, Affiliated Hospital of Guizhou Medical University (gyfynsfc-2021-41). The research procedures were approved by the Ethics Committee of the Affiliated Hospital of Guiyang Medical University [2014(87)]. All enrolled patients signed the informed consent.
Funding
Special Precision Medicine Project of the National Key R&D Program from the Science and Technology Ministry China (2016YFC0901203) and the Cultivate project 2021 for National Natural Science Foundation of China, Affiliated Hospital of Guizhou Medical University (gyfynsfc-2021-41).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
N/A.
Consent for publication
All the authors consent for the publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, R., Zhang, M., Hu, Y. et al. MiR-100-5p inhibits osteogenic differentiation of human bone mesenchymal stromal cells by targeting TMEM135. Human Cell 35, 1671–1683 (2022). https://doi.org/10.1007/s13577-022-00764-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00764-8