Abstract
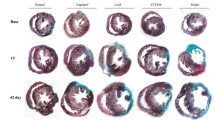
In this study, we aimed to investigate the role of SH2 domain-containing protein tyrosine phosphatase-2 (SHP-2) in cardiac remodeling after myocardial infarction (MI) and explore the underlying molecular mechanism. MI model was established by ligation of the left anterior descending coronary artery. C57/BL6J mice were randomly administered with 3.0 mg/kg/day PHPS1 (PHPS1-treated group) or normal saline (model group) by intraperitoneal injection. After 4 weeks of infusion, the effects of PHPS1 on cardiac remodeling were evaluated. Echocardiography results showed that PHPS1 treatment aggravated the MI-induced deterioration of cardiac function, with worse cardiac function parameters. PHPS1 treatment significantly increased the infarcted area, as well as the fibrotic area and the expression of collagen I and collagen III. Western blots and immunofluorescence staining showed that PHPS1 treatment up-regulated the expression of p-GRK2, p-SMAD2/3 and p-ERK1/2, while U0126 reversed the effect of PHPS1. The present study indicated that PHPS1 treatment contributed to myocardial fibrosis and infarction by activating ERK/SMAD signaling pathway, suggesting that SHP-2 may be a promising treatment target for cardiac remodeling after MI.





Similar content being viewed by others
Data availability statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Khan M, Kwiatkowski P, Rivera BK, Kuppusamy P. Oxygen and oxygenation in stem-cell therapy for myocardial infarction. Life Sci. 2010;87(9–10):269–74.
Martínez-Martínez E, Buonafine M, Boukhalfa I, Ibarrola J, Fernández-Celis A, Kolkhof P, et al. Aldosterone target NGAL (Neutrophil Gelatinase-Associated Lipocalin) is involved in cardiac remodeling after myocardial infarction through NFκB pathway. Hypertension (Dallas, Tex: 1979). 2017;70(6):1148–56. https://doi.org/10.1161/hypertensionaha.117.09791.
Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11(5):255–65. https://doi.org/10.1038/nrcardio.2014.28.
Huang S, Frangogiannis NG. Anti-inflammatory therapies in myocardial infarction: failures, hopes and challenges. Br J Pharmacol. 2018;175(9):1377–400. https://doi.org/10.1111/bph.14155.
Frangogiannis NG. The role of transforming growth factor (TGF)-β in the infarcted myocardium. J Thorac Dis. 2017;9(Suppl 1):S52–s63. https://doi.org/10.21037/jtd.2016.11.19.
Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun. 2016;7(1):1–14.
Moore-Morris T, Guimarães-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Investig. 2014;124(7):2921–34. https://doi.org/10.1172/jci74783.
Liu Y, Baumgardt SL, Fang J, Shi Y, Qiao S, Bosnjak ZJ, et al. Transgenic overexpression of GTP cyclohydrolase 1 in cardiomyocytes ameliorates post-infarction cardiac remodeling. Sci Rep. 2017;7(1):3093. https://doi.org/10.1038/s41598-017-03234-6.
Hu J, Zhang L, Zhao Z, Zhang M, Lin J, Wang J, et al. OSM mitigates post-infarction cardiac remodeling and dysfunction by up-regulating autophagy through Mst1 suppression. Biochim Biophys Acta. 2017;1863(8):1951–61. https://doi.org/10.1016/j.bbadis.2016.11.004.
Niogret C, Birchmeier W, Guarda G. SHP-2 in lymphocytes' cytokine and inhibitory receptor signaling. Front Immunol. 2019;10:2468. https://doi.org/10.3389/fimmu.2019.02468.
Tzouvelekis A, Yu G, Herazo-maya J, Xylourgidis N, Herzog E, Bennett A, et al. SH2 domain-containing phosphatase-SHP-2 is a novel regulator of fibroblast homeostasis in Pulmonary Fibrosis. QJM. 2016;109(suppl_1):S20-S.
Liu X, Li Y, Zhang Y, Lu Y, Guo W, Liu P, et al. SHP-2 promotes the maturation of oligodendrocyte precursor cells through Akt and ERK1/2 signaling in vitro. PLoS ONE. 2011;6(6):e21058. https://doi.org/10.1371/journal.pone.0021058.
Bandyopadhyay B, Han A, Dai J, Fan J, Li Y, Chen M, et al. TbetaRI/Alk5-independent TbetaRII signaling to ERK1/2 in human skin cells according to distinct levels of TbetaRII expression. J Cell Sci. 2011;124(Pt 1):19–24. https://doi.org/10.1242/jcs.076505.
Otsuka M, Goto K, Tsuchiya S, Aramaki Y. Phosphatidylserine-specific receptor contributes to TGF-beta production in macrophages through a MAP kinase. ERK Biol Pharm Bull. 2005;28(9):1707–10. https://doi.org/10.1248/bpb.28.1707.
Hao J, Ju H, Zhao S, Junaid A, Scammell-La Fleur T, Dixon IM. Elevation of expression of Smads 2, 3, and 4, decorin and TGF-beta in the chronic phase of myocardial infarct scar healing. J Mol Cell Cardiol. 1999;31(3):667–78. https://doi.org/10.1006/jmcc.1998.0902.
Odekerken JC, Walenkamp GH, Brans BT, Welting TJ, Arts JJ. The longitudinal assessment of osteomyelitis development by molecular imaging in a rabbit model. Biomed Res Int. 2014;2014:424652. https://doi.org/10.1155/2014/424652.
Patel M, Rojavin Y, Jamali AA, Wasielewski SJ, Salgado CJ. Animal models for the study of osteomyelitis. Semin Plast Surg. 2009;23(2):148–54. https://doi.org/10.1055/s-0029-1214167.
Nishiya D, Omura T, Shimada K, Matsumoto R, Kusuyama T, Enomoto S, et al. Effects of erythropoietin on cardiac remodeling after myocardial infarction. J Pharmacol Sci. 2006;101(1):31–9. https://doi.org/10.1254/jphs.fp0050966.
Shen S, Jiang H, Bei Y, Zhang J, Zhang H, Zhu H, et al. Qiliqiangxin attenuates adverse cardiac remodeling after myocardial infarction in ovariectomized mice via activation of PPARγ. Cell Physiol Biochem. 2017;42(3):876–88. https://doi.org/10.1159/000478641.
Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Fact (Chur, Switzerland). 2011;29(5):196–202. https://doi.org/10.3109/08977194.2011.595714.
Yu L, Hébert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21(14):3749–59. https://doi.org/10.1093/emboj/cdf366.
Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91. https://doi.org/10.1146/annurev.biochem.67.1.753.
Im YN, Lee YD, Park JS, Kim HK, Im SY, Song HR, et al. GPCR Kinase (GRK)-2 is a key negative regulator of itch: l-glutamine attenuates itch via a rapid induction of GRK2 in an ERK-dependent way. J Invest Dermatol. 2018;138(8):1834–42. https://doi.org/10.1016/j.jid.2018.02.036.
Zehender A, Huang J, Györfi AH, Matei AE, Trinh-Minh T, Xu X, et al. The tyrosine phosphatase SHP2 controls TGFβ-induced STAT3 signaling to regulate fibroblast activation and fibrosis. Nat Commun. 2018;9(1):3259. https://doi.org/10.1038/s41467-018-05768-3.
Fabregat I, Moreno-Càceres J, Sánchez A, Dooley S, Dewidar B, Giannelli G, et al. TGF-β signalling and liver disease. FEBS J. 2016;283(12):2219–32. https://doi.org/10.1111/febs.13665.
Fiorentini C, Savoia P, Savoldi D, Barbon A, Missale C. Persistent activation of the D1R/Shp-2/Erk1/2 pathway in l-DOPA-induced dyskinesia in the 6-hydroxy-dopamine rat model of Parkinson's disease. Neurobiol Dise. 2013;54:339–48. https://doi.org/10.1016/j.nbd.2013.01.005.
Rosário M, Birchmeier W. How to make tubes: signaling by the Met receptor tyrosine kinase. Trends Cell Biol. 2003;13(6):328–35. https://doi.org/10.1016/s0962-8924(03)00104-1.
Ivins Zito C, Kontaridis MI, Fornaro M, Feng GS, Bennett AM. SHP-2 regulates the phosphatidylinositide 3'-kinase/Akt pathway and suppresses caspase 3-mediated apoptosis. J Cell Physiol. 2004;199(2):227–36. https://doi.org/10.1002/jcp.10446.
Chen J, Cao Z, Guan J. SHP2 inhibitor PHPS1 protects against atherosclerosis by inhibiting smooth muscle cell proliferation. BMC Cardiovasc Disord. 2018;18(1):72. https://doi.org/10.1186/s12872-018-0816-2.
Salmond RJ, Alexander DR. SHP2 forecast for the immune system: fog gradually clearing. Trends Immunol. 2006;27(3):154–60. https://doi.org/10.1016/j.it.2006.01.007.
Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209(6):1201–17. https://doi.org/10.1084/jem.20112741.
Zannettino AC, Roubelakis M, Welldon KJ, Jackson DE, Simmons PJ, Bendall LJ, et al. Novel mesenchymal and haematopoietic cell isoforms of the SHP-2 docking receptor, PZR: identification, molecular cloning and effects on cell migration. Biochem J. 2003;370(Pt 2):537–49. https://doi.org/10.1042/bj20020935.
Funding
This work was supported by Prevention and Control of Geriatric Diseases in 2018 [number 2018135809-2].
Author information
Authors and Affiliations
Contributions
Conception and design, YT and YL; Data collection, YL, HT, QM and XL; Data analysis and interpretation, JC and XZ; Drafting article, YL, XL and YT; Administrative support, YT. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All animal experiments were performed in accordance with the guidelines for animal care and the experimental protocols were approved by the Institutional Animal Care and Use Committee of our hospital.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, YG., Tan, H., Ma, Q. et al. SH2 domain-containing protein tyrosine phosphatase-2 (SHP-2) prevents cardiac remodeling after myocardial infarction through ERK/SMAD signaling pathway. Human Cell 34, 325–334 (2021). https://doi.org/10.1007/s13577-020-00430-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00430-x