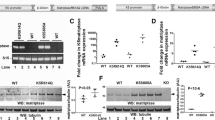
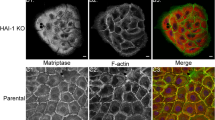
Abstract
Studies of human genetic disorders and animal models indicate that matriptase plays essential roles in proteolytic processes associated with profilaggrin processing and desquamation at late stages of epidermal differentiation. The tissue distribution profile and zymogen activation status in human skin, however, suggests that matriptase physiological function in the skin more likely lies in the proliferating and differentiating keratinocytes in the basal and spinous layers. Marked acanthosis with expanded spinous layer and lack of significant changes in intensity and expression pattern for several terminal differentiation markers in the skin of ARIH patients support matriptase’s role in earlier rather than the later stages of differentiation. In addition to the tissue distribution, differential subcellular localization further limits the ability of extracellular matriptase proteolytic activity to access the cytosolic non-membrane-bound keratohyalin granules, in which profilaggrin processing occurs. The short lifespan of active matriptase, which results from tightly controlled zymogen activation, rapid inhibition by HAI-1, and shedding from cell surface, indicates that active matriptase likely performs physiological functions via limited proteolysis on its substrates, as needed, rather than via a continuous bulk process. We, here, review these spatiotemporal controls of matriptase proteolytic activity at the biochemical, cellular, and tissue level. Based on this in-depth understanding of how matriptase activity is regulated, we argue that there is no direct involvement of matriptase proteolytic activity in profilaggrin processing and desquamation. The defects in epidermal terminal differentiation associated with matriptase deficiency are likely secondary and are due to putative disruption at earlier stages of differentiation.
Similar content being viewed by others
References
List K, Haudenschild CC, Szabo R, Chen W, Wahl SM, Swaim W, Engelholm LH, Behrendt N, Bugge TH. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene. 2002;21:3765–79.
List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T, Burke B, Nielsen BS, Gutkind JS, Bugge TH. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev. 2005;19:1934–50.
Kim MG, Chen C, Lyu MS, Cho EG, Park D, Kozak C, Schwartz RH. Cloning and chromosomal mapping of a gene isolated from thymic stromal cells encoding a new mouse type II membrane serine protease, epithin, containing four LDL receptor modules and two CUB domains. Immunogenetics. 1999;49:420–8.
Lin CY, Anders J, Johnson M, Sang QA, Dickson RB. Molecular cloning of cDNA for matriptase, a matrix-degrading serine protease with trypsin-like activity. J Biol Chem. 1999;274:18231–6.
Takeuchi T, Shuman MA, Craik CS. Reverse biochemistry: use of macromolecular protease inhibitors to dissect complex biological processes and identify a membrane-type serine protease in epithelial cancer and normal tissue. Proc Natl Acad Sci USA. 1999;96:11054–61.
Lee SL, Dickson RB, Lin CY. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem. 2000;275:36720–5.
Takeuchi T, Harris JL, Huang W, Yan KW, Coughlin SR, Craik CS. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem. 2000;275:26333–42.
List K, Szabo R, Wertz PW, Segre J, Haudenschild CC, Kim SY, Bugge TH. Loss of proteolytically processed filaggrin caused by epidermal deletion of Matriptase/MT-SP1. J Cell Biol. 2003;163:901–10.
Chen CJ, Wu BY, Tsao PI, Chen CY, Wu MH, Chan YL, Lee HS, Johnson MD, Eckert RL, Chen YW, Chou F, Wang JK, Lin CY. Increased matriptase zymogen activation in inflammatory skin disorders. Am J Physiol Cell Physiol. 2011;300:C406–415.
Chen YW, Wang JK, Chou FP, Wu BY, Hsiao HC, Chiu H, Xu Z, Baksh ANH, Shi G, Kaul M, Barndt R, Shanmugam V, Johnson M, Lin CY. Matriptase regulates proliferation and early, but not terminal, differentiation of human keratinocytes. J Invest Dermatol. 2013;134:405–14.
Wu BY, Lee SP, Hsiao HC, Chiu H, Chen CY, Yeo YH, Lee HS, Chen YW, Kaul M, Kataoka H, Johnson MD, Wang JK, Lin CY. Matriptase expression and zymogen activation in human pilosebaceous unit. J Histochem Cytochem. 2013;62:50–9.
Lai CH, Chang SC, Chen YJ, Wang YJ, Lai YJ, Chang HD, Berens EB, Johnson MD, Wang JK, Lin CY. Matriptase and prostasin are expressed in human skin in an inverse trend over the course of differentiation and are targeted to different regions of the plasma membrane. Biol Open. 2016;15:1380–7.
Lee SP, Kao CY, Chang SC, Chiu YL, Chen YJ, Chen MG, Chang CC, Lin YW, Chiang CP, Wang JK, Lin CY, Johnson MD. Tissue distribution and subcellular localizations determine in vivo functional relationship among prostasin, matriptase, HAI-1, and HAI-2 in human skin. PLoS ONE. 2018;13:e0192632.
Friedrich R, Fuentes-Prior P, Ong E, Coombs G, Hunter M, Oehler R, Pierson D, Gonzalez R, Huber R, Bode W, Madison EL. Catalytic domain structures of MT-SP1/matriptase, a matrix-degrading transmembrane serine proteinase. J Biol Chem. 2002;277:2160–8.
Lin CY, Anders J, Johnson M, Dickson RB. Purification and characterization of a complex containing matriptase and a Kunitz-type serine protease inhibitor from human milk. J Biol Chem. 1999;274:18237–42.
Oberst MD, Chen LY, Kiyomiya KI, Williams CA, Lee MS, Johnson MD, Dickson RB, Lin CY. Hepatocyte growth factor activator inhibitor 1 (HAI-1) regulates activation and expression of matriptase, a membrane-bound serine protease. Am J Physiol Cell Physiol. 2005;289:C462–C470470.
Sah RP, Saluja AK. Trypsinogen activation in acute and chronic pancreatitis: is it a prerequisite? Gut. 2011;60:1305–7.
Oberst MD, Williams CA, Dickson RB, Johnson MD, Lin CY. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J Biol Chem. 2003;278:26773–9.
Khan AR, James MN. Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Protein Sci. 1998;7:815–36.
Morgan PH, Robinson NC, Walsh KA, Neurath H. Inactivation of bovine trypsinogen and chymotrypsinogen by diisopropylphosphorofluoridate. Proc Natl Acad Sci USA. 1972;69:3312–6.
Lamba D, Bauer M, Huber R, Fischer S, Rudolph R, Kohnert U, Bode W. The 2.3 A crystal structure of the catalytic domain of recombinant two-chain human tissue-type plasminogen activator. J Mol Biol. 1996;258:117–35.
Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP. Control of the complement system. Adv Immunol. 1996;61:201–83.
Jin X, Hirosaki T, Lin CY, Dickson RB, Higashi S, Kitamura H, Miyazaki K. Production of soluble matriptase by human cancer cell lines and cell surface activation of its zymogen by trypsin. J Cell Biochem. 2005;95:632–47.
Buzza MS, Martin EW, Driesbaugh KH, Desilets A, Leduc R, Antalis TM. Prostasin is required for matriptase activation in intestinal epithelial cells to regulate closure of the paracellular pathway. J Biol Chem. 2013;288:10328–37.
Camerer E, Barker A, Duong DN, Ganesan R, Kataoka H, Cornelissen I, Darragh MR, Hussain A, Zheng YW, Srinivasan Y, Brown C, Xu SM, Regard JB, Lin CY, Craik CS, Kirchhofer D, Coughlin SR. Local protease signaling contributes to neural tube closure in the mouse embryo. Dev Cell. 2010;18:25–38.
Ko CJ, Huang CC, Lin HY, Juan CP, Lan SW, Shyu HY, Wu SR, Hsiao PW, Huang HP, Shun CT, Lee MS. Androgen-Induced TMPRSS2 activates matriptase and promotes extracellular matrix degradation, prostate cancer cell invasion, tumor growth, and metastasis. Cancer Res. 2015;75:2949–60.
Benaud C, Dickson RB, Lin CY. Regulation of the activity of matriptase on epithelial cell surfaces by a blood-derived factor. Eur J Biochem. 2001;268:1439–47.
Benaud C, Oberst M, Hobson JP, Spiegel S, Dickson RB, Lin CY. Sphingosine 1-phosphate, present in serum-derived lipoproteins, activates matriptase. J Biol Chem. 2002;277:10539–46.
Hung RJ, Hsu I, Dreiling JL, Lee MJ, Williams CA, Oberst MD, Dickson RB, Lin CY. Assembly of adherens junctions is required for sphingosine 1-phosphate-induced matriptase accumulation and activation at mammary epithelial cell-cell contacts. Am J Physiol Cell Physiol. 2004;286:C1159–C1169169.
Lee M-S, Kiyomiya K, Benaud C, Dickson RB, Lin CY. Simultaneous activation and HAI-1-mediated inhibition of matriptase induced at activation foci in immortal human mammary epithelial cells. Am J Physiol Cell Physiol. 2005;288:C932–C941941.
Kiyomiya KI, Lee MS, Tseng IC, Zuo H, Barndt RJ, Johnson MD, Dickson RB, Lin CY. Matriptase activation and subsequent shedding with HAI-1 is induced by steroid sex hormones in human prostate cancer cells, but not in breast cancer cells. Am J Physiol Cell Physiol. 2006;291:C40–C4949.
Chen YW, Yin S, Lai YJ, Johnson MD, Lin CY. Plasminogen-dependent matriptase activation accelerates plasmin generation by differentiating primary human keratinocytes. J Invest Dermatol. 2016;136:1210–8.
Lee MS, Tseng IC, Wang Y, Kiyomiya K, Johnson MD, Dickson RB, Lin CY. Autoactivation of matriptase in vitro: requirement for biomembrane and LDL receptor domain. Am J Physiol Cell Physiol. 2007;293:C95–C105.
Tseng IC, Xu H, Chou FP, Li G, Vazzano AP, Kao JP, Johnson MD, Lin CY. Matriptase activation, an early cellular response to acidosis. J Biol Chem. 2010;285:3261–70.
Wang JK, Teng IJ, Lo TJ, Moore S, Yeo YH, Teng YC, Kaul M, Chen CC, Zuo AH, Chou FP, Yang X, Tseng IC, Johnson MD, Lin CY. Matriptase autoactivation is tightly regulated by the cellular chemical environments. PLoS ONE. 2014;9:e93899.
Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–39.
Chen YW, Wang JK, Chou FP, Chen CY, Rorke EA, Chen LM, Chai KX, Eckert RL, Johnson MD, Lin CY. Regulation of the matriptase-prostasin cell surface proteolytic cascade by hepatocyte growth factor activator inhibitor-1 (HAI-1) during epidermal differentiation. J Biol Chem. 2010;285:31755–62.
Tseng CC, Jia B, Barndt R, Gu Y, Chen CY, Tseng IC, Su SF, Wang JK, Johnson MD, Lin CY. Matriptase shedding is closely coupled with matriptase zymogen activation and requires de novo proteolytic cleavage likely involving its own activity. PLoS ONE. 2017;12:e0183507.
Kilpatrick LM, Harris RL, Owen KA, Bass R, Ghorayeb C, Bar-Or A, Ellis V. Initiation of plasminogen activation on the surface of monocytes expressing the type II transmembrane serine protease matriptase. Blood. 2006;108:2616–23.
Chou FP, Chen YW, Zhao XF, Xu-Monette ZY, Gartenhaus RB, Wang JK, Kataoka H, Zhou AH, Barndt R, Johnsen M, Lin CY. Imbalanced matriptase pericellular proteolysis contributes to the pathogenesis of malignant B-cell lymphomas. Am J Pathol. 2013;183:1306–17.
Panteleyev AA, Jahoda CA, Christiano AM. Hair follicle predetermination. J Cell Sci. 2001;114:3419–31.
Chu LL, Xu Y, Yang JR, Hu YA, Chang HH, Lai HY, Tseng CC, Wang HY, Johnson MD, Wang JK, Lin CY. Human cancer cells retain modest levels of enzymatically active matriptase only in extracellular milieu following induction of zymogen activation. PLoS ONE. 2014;9:e92244.
Basel-Vanagaite L, Attia R, Ishida-Yamamoto A, Rainshtein L, Ben AD, Lurie R, Pasmanik-Chor M, Indelman M, Zvulunov A, Saban S, Magal N, Sprecher E, Shohat M. Autosomal recessive ichthyosis with hypotrichosis caused by a mutation in ST14, encoding type II transmembrane serine protease matriptase. Am J Hum Genet. 2007;80:467–77.
Avrahami L, Maas S, Pasmanik-Chor M, Rainshtein L, Magal N, Smitt J, van MJ, Shohat M, Basel-Vanagaite L. Autosomal recessive ichthyosis with hypotrichosis syndrome: further delineation of the phenotype. Clin Genet. 2008;74:47–53.
Alef T, Torres S, Hausser I, Metze D, Tursen U, Lestringant GG, Hennies HC. Ichthyosis, follicular atrophoderma, and hypotrichosis caused by mutations in ST14 is associated with impaired profilaggrin processing. J Invest Dermatol. 2009;129:862–9.
Desilets A, Beliveau F, Vandal G, McDuff FO, Lavigne P, Leduc R. Mutation G827R in matriptase causing autosomal recessive ichthyosis with hypotrichosis yields an inactive protease. J Biol Chem. 2008;283:10535–42.
Ito M. Electron microscopic study on cell differentiation in anagen hair follicles in mice. J Invest Dermatol. 1988;90:65–72.
Cho EG, Kim MG, Kim C, Kim SR, Seong IS, Chung C, Schwartz RH, Park D. N-terminal processing is essential for release of epithin, a mouse type II membrane serine protease. J Biol Chem. 2001;276:44581–9.
Zoratti GL, Tanabe LM, Varela FA, Murray AS, Bergum C, Colombo E, Lang JE, Molinolo AA, Leduc R, Marsault E, Boerner J, List K. Targeting matriptase in breast cancer abrogates tumour progression via impairment of stromal-epithelial growth factor signalling. Nat Commun. 2015;6:6776.
Netzel-Arnett S, Currie BM, Szabo R, Lin CY, Chen LM, Chai KX, Antalis TM, Bugge TH, List K. Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. J Biol Chem. 2006;281:32941–5.
Szabo R, Lantsman T, Peters DE, Bugge TH. Delineation of proteolytic and non-proteolytic functions of membrane-anchored serine protease Prss8/prostasin. Development. 2016;143:2818–28.
Yu JX, Chao L, Chao J. Prostasin is a novel human serine proteinase from seminal fluid. Purification, tissue distribution, and localization in prostate gland. J Biol Chem. 1994;269:18843–8.
Friis S, Sales KU, Godiksen S, Peters DE, Lin CY, Vogel LK, Bugge TH. A matriptase-prostasin reciprocal zymogen activation complex with unique features: prostasin as a non-enzymatic co-factor for matriptase activation. J Biol Chem. 2013;288:19028–39.
List K, Currie B, Scharschmidt TC, Szabo R, Shireman J, Molinolo A, Cravatt BF, Segre J, Bugge TH. Autosomal ichthyosis with hypotrichosis syndrome displays low matriptase proteolytic activity and is phenocopied in ST14 hypomorphic mice. J Biol Chem. 2007;282:36714–23.
List K, Szabo R, Molinolo A, Nielsen BS, Bugge TH. Delineation of matriptase protein expression by enzymatic gene trapping suggests diverging roles in barrier function, hair formation, and squamous cell carcinogenesis. Am J Pathol. 2006;168:1513–25.
Nagaike K, Kawaguchi M, Takeda N, Fukushima T, Sawaguchi A, Kohama K, Setoyama M, Kataoka H. Defect of hepatocyte growth factor activator inhibitor type 1/serine protease inhibitor, kunitz type 1 (Hai-1/Spint1) leads to ichthyosis-like condition and abnormal hair development in mice. Am J Pathol. 2008;173:1464–75.
Sales KU, Masedunskas A, Bey AL, Rasmussen AL, Weigert R, List K, Szabo R, Overbeek PA, Bugge TH. Matriptase initiates activation of epidermal pro-kallikrein and disease onset in a mouse model of Netherton syndrome. Nat Genet. 2010;42:676–83.
Acknowledgements
This study was supported by National Cancer Institute (NCI) Grant RO1 CA 123223 (to MDJ and CYL), and Grant (MAB-108-079) from the Ministry of National Defense Medical Affairs Bureau, Taiwan and Grants (CMNDMC10705; CMNDMC10813) from Chi-Mei Medical Center, Tainan, Taiwan (to J.-K. Wang). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CYL is an inventor on US patents #6,077,938 (Title: Monoclonal antibody to an 80-kDa protease) and #6,677,377 (Title: Structure based discovery of inhibitors of matriptase for the cancer diagnosis and therapy by detection and inhibition of matriptase activity) and MDJ and CYL are inventors on US patent #7,355,015 (Title: Matriptase, a serine protease and its applications). This does not alter our adherence to the journal policies on sharing data and materials and official views of the National Cancer Institute or the National Institutes of Health.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lin, CY., Wang, JK. & Johnson, M.D. The spatiotemporal control of human matriptase action on its physiological substrates: a case against a direct role for matriptase proteolytic activity in profilaggrin processing and desquamation. Human Cell 33, 459–469 (2020). https://doi.org/10.1007/s13577-020-00361-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00361-7