Abstract
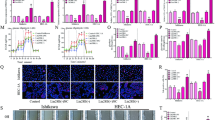
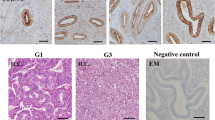
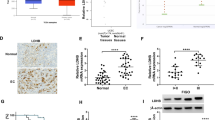
Anterior gradient 2 (AGR2) was proved to modulate cancer progression. However, the role of AGR2 on endometrial cancer was not established. Here, we investigated the effects of AGR2 expression on endometrial cancer and explored the regulation mechanism. In the study, we found that AGR2 was overexpressed in tumor tissues of 30 endometrial cancer patients. A high level of AGR2 promoted endometrial cancer cells proliferation, migration and invasion. AGR2 induced the expression of lactate dehydrogenase A (LDHA), phosphoglycerate kinase 1 (PGK1), kallikrein 2 (HK2), and enolase 1-α (ENO1), glucose uptake and lactate production. AGR2 could bind to MUC1 and induce MUC1 and hypoxia-inducible factor 1α (HIF-1α). The inhibition effects of AGR2 knockdown on cells proliferation, migration and invasion ability were abolished by the overexpression of MUC1. Besides, the overexpression of MUC1 also reversed the inhibition effects of AGR2 knockdown on the expression of LDHA, HK2, PGK1 and ENO1, glucose uptake and lactate production. AGR2 knockdown inhibited tumor growth, the levels of Ki-67, MUC1, HIF-1α and glycolysis. In conclusion, AGR2 was overexpressed in endometrial cancer and AGR2-induced glucose metabolism facilitated the progression of endometrial carcinoma via the MUC1/HIF-1α pathway. AGR2 may be an effective therapeutic target for endometrial carcinoma.





Similar content being viewed by others
References
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet (Lond Engl). 2016;387(10023):1094–108. https://doi.org/10.1016/s0140-6736(15)00130-0.
Al-Maghrabi J, Abdelrahman A, Al-Maghrabi B, Buhmeida A, Abuzenadah A, Al-Qahtani M, et al. Loss of p27 expression in endometrial carcinoma patients with recurrent tumor is significantly associated with poor survival. Eur J Gynaecol Oncol. 2018;39(1):119–23.
Abdulfatah E, Ahmed Q, Alosh B, Bandyopadhyay S, Bluth MH, Ali-Fehmi R. Gynecologic cancers: molecular updates 2018. Clin Lab Med. 2018;38(2):421–38. https://doi.org/10.1016/j.cll.2018.02.007.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. https://doi.org/10.3322/caac.21387.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. https://doi.org/10.3322/caac.21166.
Vale CL, Tierney J, Bull SJ, Symonds PR. Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma. Cochrane Database Syst Rev. 2012;8:CD003915. https://doi.org/10.1002/14651858.CD003915.pub4.
Persson S, Rosenquist M, Knoblach B, Khosravi-Far R, Sommarin M, Michalak M. Diversity of the protein disulfide isomerase family: identification of breast tumor induced Hag2 and Hag3 as novel members of the protein family. Mol Phylogenet Evol. 2005;36(3):734–40. https://doi.org/10.1016/j.ympev.2005.04.002.
Alavi M, Mah V, Maresh EL, Bagryanova L, Horvath S, Chia D, et al. High expression of AGR2 in lung cancer is predictive of poor survival. BMC Cancer. 2015;15:655. https://doi.org/10.1186/s12885-015-1658-2.
Zhang J, Jin Y, Xu S, Zheng J, Zhang QI, Wang Y, et al. AGR2 is associated with gastric cancer progression and poor survival. Oncol Lett. 2016;11(3):2075–83. https://doi.org/10.3892/ol.2016.4160.
Kyoungsook P, Jin CY, Hyekyung S, Kwangsoo K, Junsoo P, Mijoung O, et al. AGR2, a mucinous ovarian cancer marker, promotes cell proliferation and migration. Exp Mol Med. 2011;43(2):91–100.
Jung SY, Yun J, Kim SJ, Kang S, Kim DY, Kim YJ, et al. Basic helix-loop-helix transcription factor Twist1 is a novel regulator of anterior gradient protein 2 homolog (AGR2) in breast cancer. Biochem Biophys Res Commun. 2019;516(1):149–56. https://doi.org/10.1016/j.bbrc.2019.05.191.
Kamal A, Valentijn A, Barraclough R, Rudland P, Rahmatalla N, Martin-Hirsch P, et al. High AGR2 protein is a feature of low grade endometrial cancer cells. Oncotarget. 2018;9(59):31459–72. https://doi.org/10.18632/oncotarget.25838.
Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70(1):431–57.
Ren J, Raina D, Chen W, Li G, Huang L, Kufe D. MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol Cancer Res Mcr. 2006;4(11):873.
Singh PK, Yunfei W, Swanson BJ, Kandavel S, Andrius K, Cerny RL, et al. Platelet-derived growth factor receptor beta-mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res. 2007;67(11):5201–10.
McGuckin MA, Walsh MD, Hohn BG, Ward BG, Wright RG. Prognostic significance of MUC1 epithelial mucin expression in breast cancer. Hum Pathol. 1995;26(4):432–9. https://doi.org/10.1016/0046-8177(95)90146-9.
Hebbar V, Damera G, Sachdev GP. Differential expression of MUC genes in endometrial and cervical tissues and tumors. BMC Cancer. 2005;5(1):124.
Engel BJ, Bowser JL, Broaddus RR, Carson DD. MUC1 stimulates EGFR expression and function in endometrial cancer. Oncotarget. 2016;7(22):32796–809.
Shukla SK, Purohit V, Mehla K, Gunda V, Chaika NV, Vernucci E, et al. MUC1 and HIF-1alpha signaling crosstalk induces anabolic glucose metabolism to impart gemcitabine resistance to pancreatic cancer. Cancer Cell. 2017;32(1):71–87.e7.
Huang J, Liu L, Feng M, An S, Zhou M, Li Z, et al. Effect of CoCl2 on fracture repair in a rat model of bone fracture. Mol Med Rep. 2015;12(4):5951–6. https://doi.org/10.3892/mmr.2015.4122.
Chaika NV, Gebregiworgis T, Lewallen ME, Purohit V, Radhakrishnan P, Liu X, et al. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc Natl Acad Sci USA. 2012;109(34):13787–92. https://doi.org/10.1073/pnas.1203339109.
Prat J. Pathology of cancers of the female genital tract. Int J Gynaecol Obst. 2015;131(Suppl 2):S132–S145145. https://doi.org/10.1016/j.ijgo.2015.06.010.
Ma SR, Mao L, Deng WW, Li YC, Bu LL, Yu GT, et al. AGR2 promotes the proliferation, migration and regulates epithelial-mesenchymal transition in salivary adenoid cystic carcinoma. Am J Transl Res. 2017;9(2):507–19.
Li Y, Wang W, Liu Z, Jiang Y, Lu J, Xie H, et al. AGR2 diagnostic value in nasopharyngeal carcinoma prognosis. Clin Chim Acta Int J Clin Chem. 2018;484:323–7. https://doi.org/10.1016/j.cca.2017.12.023.
Fritzsche FR, Dahl E, Dankof A, Burkhardt M, Pahl S, Petersen I et al (2007) Expression of AGR2 in non small cell lung cancer. Histol Histopathol
Milewski D, Balli D, Ustiyan V, Le T, Dienemann H, Warth A, et al. FOXM1 activates AGR2 and causes progression of lung adenomas into invasive mucinous adenocarcinomas. PLoS Genet. 2017;13(12):e1007097.
Wang Z, Hao Y, Lowe AW. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res. 2008;68(2):492–7.
Annibaldi A, Widmann C. Glucose metabolism in cancer cells. Curr Opin Clin Nutr Metab Care. 2010;13(4):466–70.
Kufe DW. Functional targeting of the MUC1 oncogene in human cancers. Cancer Biol Ther. 2009;8(13):1197–203.
Li Y, Liu D, Chen D, Kharbanda S, Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22(38):6107.
Norris AM, Gore A, Balboni A, Young A, Longnecker DS, Korc M. AGR2 is a SMAD4-suppressible gene that modulates MUC1 levels and promotes the initiation and progression of pancreatic intraepithelial neoplasia. Oncogene. 2013;32(33):3867–76. https://doi.org/10.1038/onc.2012.394.
Mehla K, Singh PK. MUC1: a novel metabolic master regulator. Biochim Biophys Acta (BBA) Rev Cancer 2014;1845(2):126–135
Kosugi M, Ahmad R, Alam M, Uchida Y, Kufe D. MUC1-C oncoprotein regulates glycolysis and pyruvate kinase M2 activity in cancer cells. PLoS One. 2011;6(11):e28234.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
WG conceived and designed the experiments, BE and XW analyzed and interpreted the results of the experiments, and YL and LW performed the experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests, and all authors would confirm its accuracy.
Ethics approval and consent to participate
The protocol of the study was approved by the Ethics Committee of The Fourth Affiliated Hospital of Xinjiang Medical University (No. 2017065), and all the patients signed written informed consent.
All animal experiments were approved by the Ethics Committee of The Fourth Affiliated Hospital of Xinjiang Medical University (No. 2017065).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Patient consent for publication
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gong, W., Ekmu, B., Wang, X. et al. AGR2-induced glucose metabolism facilitated the progression of endometrial carcinoma via enhancing the MUC1/HIF-1α pathway. Human Cell 33, 790–800 (2020). https://doi.org/10.1007/s13577-020-00356-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-020-00356-4