Abstract
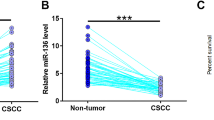
This study aimed to investigate the role of lncRNA terminal differentiation-induced ncRNA (TINCR) in cervical squamous cell carcinoma (CSCC). By informatics analysis, we found that miR-302 may bind TINCR. Expression analysis showed that miR-302 was downregulated, while TINCR was upregulated in CSCC. Correlation analysis showed that they were not significantly correlated. In CSCC cells, miR-302 and TINCR failed to affect the expression of each other. However, miR-302 overexpression led to downregulated and TINCR overexpression led to upregulated cyclin D1 expression in CSCC cells. Interestingly, overexpression of cyclin D1 led to upregulated miR-302 and TINCR. Cell proliferation analysis showed that TINCR and cyclin D1 overexpression led to increased, while miR-302 overexpression led to decreased rate of cell proliferation. Moreover, miR-302 overexpression reduced the effects of TINCR overexpression. Therefore, TINCR sponges miR-302 to upregulate cyclin D1 in CSCC, thereby promoting cell proliferation.





Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. https://doi.org/10.3322/caac.21551.
Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23. https://doi.org/10.1016/j.vaccine.2012.07.055.
Groves IJ, Coleman N. Pathogenesis of human papillomavirus-associated mucosal disease. J Pathol. 2015;235(4):527–38. https://doi.org/10.1002/path.4496.
Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27. https://doi.org/10.1056/NEJMoa021641.
Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88(1):63–73. https://doi.org/10.1038/sj.bjc.6600688.
Stanley MA, Sudenga SL, Giuliano AR. Alternative dosage schedules with HPV virus-like particle vaccines. Expert Rev Vaccines. 2014;13(8):1027–38. https://doi.org/10.1586/14760584.2014.935767.
Anastasiadou E, Jacob LS, Slack FJ. Non-coding RNA networks in cancer. Nat Rev Cancer. 2018;18(1):5–18. https://doi.org/10.1038/nrc.2017.99.
Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer—a brief overview. Adv Biol Regul. 2015;57:1–9. https://doi.org/10.1016/j.jbior.2014.09.013.
Schmitt AM, Chang HY. Long non-coding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–63. https://doi.org/10.1016/j.ccell.2016.03.010.
Yoon JH, Abdelmohsen K, Gorospe M. Functional interactions among microRNAs and long noncoding RNAs. Semin Cell Dev Biol. 2014;34(4):9–14.
Chen Z, Liu Y, He A, Li J, Chen M, Zhan Y, et al. Theophylline controllable RNAi-based genetic switches regulate expression of lncRNA TINCR and malignant phenotypes in bladder cancer cells. Sci Rep. 2016;6:30798. https://doi.org/10.1038/srep30798.
Zhang ZY, Lu YX, Zhang ZY, Chang YY, Zheng L, Yuan L, et al. Loss of TINCR expression promotes proliferation, metastasis through activating EpCAM cleavage in colorectal cancer. Oncotarget. 2016;7(16):22639–49. https://doi.org/10.18632/oncotarget.8141.
Card DA, Hebbar PB, Li L, Trotter KW, Komatsu Y, Mishina Y, et al. Oct4/Sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol Cell Biol. 2008;28(20):6426–38. https://doi.org/10.1128/MCB.00359-08.
Dong H, Hu J, Zou K, Ye M, Chen Y, Wu C, et al. Activation of LncRNA TINCR by H3K27 acetylation promotes Trastuzumab resistance and epithelial–mesenchymal transition by targeting MicroRNA-125b in breast Cancer. Mol Cancer. 2019;18(1):3. https://doi.org/10.1186/s12943-018-0931-9.
Hausser J, Zavolan M. Identification and consequences of miRNA-target interactions-beyond repression of gene expression. Nat Rev Genet. 2014;15(9):599–612. https://doi.org/10.1038/nrg3765.
Liang WC, Fu WM, Wong CW, Wang Y, Wang WM, Hu GX, et al. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget. 2015;6(26):22513–25. https://doi.org/10.18632/oncotarget.4154.
Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing miRNA–lncRNA interactions. Methods Mol Biol. 2016;1402:271–86. https://doi.org/10.1007/978-1-4939-3378-5_21.
Faherty N, Curran SP, O’Donovan H, Martin F, Godson C, Brazil DP, et al. CCN2/CTGF increases expression of miR-302 microRNAs, which target the TGFbeta type II receptor with implications for nephropathic cell phenotypes. J Cell Sci. 2012;125(Pt 23):5621–9. https://doi.org/10.1242/jcs.105528.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
University of Chinese Academy of Sciences Shenzhen Hospital Ethics Committee approved this study. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hou, A., Zhang, Y., Zheng, Y. et al. LncRNA terminal differentiation-induced ncRNA (TINCR) sponges miR-302 to upregulate cyclin D1 in cervical squamous cell carcinoma (CSCC). Human Cell 32, 515–521 (2019). https://doi.org/10.1007/s13577-019-00268-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-019-00268-y