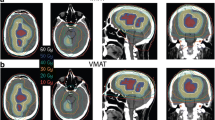
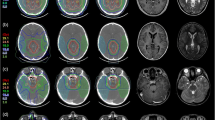
Abstract
Background
Stereotactic radiosurgery (SRS), stereotactic body radiotherapy (SBRT), and stereotactic ablative body radiotherapy (SABR) are commonly used in the treatment of central nervous system (CNS) disease. This study has refined the radiation toxicity estimates for some normal tissues of the CNS based on review and analysis of the clinical evidence for single fraction radiosurgery, hypofractionated SBRT, and conventionally fractionated radiation therapy.
Methods
Published guidelines and protocols are reviewed. In the past, many normal tissue tolerances were compiled based on the experience of the investigators and publications in the literature. Some tolerances were determined by modeling or calculation using the existing biological formulas, in particular the linear quadratic (LQ) model. In the present study, the estimate of risk for each dose tolerance limit in some CNS tissues is provided exclusively with normal tissue complication probability (NTCP). The clinical outcomes are compared to understand the difference in biological effect between radiosurgery and radiotherapy.
Results
Normal tissue dose tolerances and the corresponding complication rates are provided for brainstem, optic nerves, cochlea, and spinal cord, including single fraction SRS, five-fraction SBRT, and conventional radiation therapy. Calculation of biologically effective dose (BED) or single fraction equivalent dose (SFED) alone using the LQ model conveys no consensus on the biological effect across different fractionations. Comparison of conventional radiation therapy to brain and spinal cord with single fraction equivalent dose leads to even conflicting clinical outcomes.
Conclusions
Effective differences between single fraction SRS and conventional radiotherapy need to be better understood. The existing biological model might not be valid to predict the radiosurgical outcomes based on conventionally fractionated radiotherapy. However, application of the statistical dose response models of clinical SRS and SBRT outcomes data to selected current dose tolerance guidelines into simple tables can be a clinically useful resource.


Similar content being viewed by others
Notes
The Hindo et al. study [90] of parallel opposed whole brain fields during the era of Cobalt differs in many ways from the modern era of radiosurgery, especially in terms of patient selection, prognosis, dose distribution, and other factors. Perhaps the most relevant comparison is for brainstem, since the entire cross-section of brainstem was probably within ± 10% of a comparable physical dose of 10 Gy/1 fraction in the whole brain study, whereas for modern radiosurgery only a small volume of the critical structure is usually allowed to reach levels like 10 Gy in 1 fraction. We are not advocating 10 Gy in 1 fraction to a large volume, but we feel it is an interesting historical point of comparison.
Abbreviations
- AE:
-
Adverse event
- BED:
-
Biologically effective dose
- CNS:
-
Central nervous system
- GTV:
-
Gross tumor volume
- HyTEC:
-
High Dose per Fraction, Hypofractionated Treatment Effects in the Clinic
- LQ:
-
Linear quadratic
- MLE:
-
Maximum likelihood estimate
- NfxED:
-
N-fraction equivalent dose
- NTCP:
-
Normal tissue complication probability
- QUANTEC:
-
Quantitative Analysis of Normal Tissue Effects in the Clinic
- RT:
-
Radiation therapy
- RTOG:
-
Radiation therapy oncology group
- SABR:
-
Stereotactic ablative body radiotherapy
- SBRT:
-
Stereotactic body radiation therapy
- SFED:
-
Single fraction equivalent dose
- SRS:
-
Stereotactic radiosurgery
- WBRT:
-
Whole brain radiation therapy
References
Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M (1991) Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 21(1):109–122
Emami B, Roeske J, Block A, Welsh JS (2017) The Emami paper 25 years later. J Radiat Oncol 6(1):1–9
Marks LB, Ten Haken RK, Martel MK (2010) Guest editor’s introduction to QUANTEC: a users guide. Int J Radiat Oncol Biol Phys 76(3 Suppl):S1–S2
Asbell SO, Grimm J, Xue J, Chew MS, LaCouture TL (2016) Introduction and clinical overview of the DVH risk map. Semin Radiat Oncol 26(2):89–96
Grimm J, Sahgal A, Soltys SG, Luxton G, Patel A, Herbert S, Xue J, Ma L, Yorke E, Adler JR, Gibbs IC (2016) Estimated risk level of unified stereotactic body radiation therapy dose tolerance limits for spinal cord. Semin Radiat Oncol 26(2):165–171
Hiniker SM, Modlin LA, Choi CY, Atalar B, Seiger K, Binkley MS, Harris JP, Liao YJ, Fischbein N, Wang L, Ho A, Lo A, Chang SD, Harsh GR, Gibbs IC, Hancock SL, Li G, Adler JR, Soltys SG (2016) Dose-response modeling of the visual pathway tolerance to single-fraction and hypofractionated stereotactic radiosurgery. Semin Radiat Oncol 26(2):97–104
Rashid A, Karam SD, Rashid B, Kim JH, Pang D, Jean W, Grimm J, Collins SP (2016) Multisession radiosurgery for hearing preservation. Semin Radiat Oncol 26(2):105–111
Coia L, Galvin J, Sontag M, Blitzer P, Brenner H, Cheng E, Doppke K, Harms W, Hunt M, Mohan R, Munzenrider J, Simpson J (1991) Three-dimensional photon treatment planning in carcinoma of the larynx. Int J Radiat Oncol Biol Phys 21(1):183–192
Simpson JR, Purdy JA, Manolis JM, Pilepich MV, Burman C, Forman J, Fuks Z, Cheng E, Chu J, Matthews J, Mohan R, Solin L, Tepper J, Urie M (1991) Three-dimensional treatment planning considerations for prostate cancer. Int J Radiat Oncol Biol Phys 21(1):243–252
Shank B, LoSasso T, Brewster L, Burman C, Cheng E, Chu JC, Drzymala RE, Manolis J, Pilepich MV, Solin LJ, Tepper JE, Urie M (1991) Three-dimensional treatment planning for postoperative treatment of rectal carcinoma. Int J Radiat Oncol Biol Phys 21(1):253–265
Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, Bentzen SM, Nam J, Deasy JO (2010) Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 76(3 Suppl):S10–S19
Milano MT, Grimm J, Soltys SG, Yorke E, Moiseenko V, Tomé WA, Sahgal A, Xue J, Ma L, Solberg T, Kirkpatrick JP, Constine LS, Flickinger JC, Marks LB, El Naqa I. Single and multi-fraction stereotactic radiosurgery dose tolerances of the optic pathways. Int J Radiat Oncol Biol Phys. [HyTEC issue, In Press]
Vargo JA, Moiseenko V, Grimm J, Caudell J, Clump DA, Yorke E, Xue J, Vinogradskiy Y, Moros EG, Mavroidis P, Jain S, El Naqa I, Marks LB, Heron DE. Head and neck tumor control probability: radiation dose-volume effects in SBRT for locally-recurrent previously-irradiated head and neck cancer. Int J Radiat Oncol Biol Phys. [HyTEC issue, In Press]
Ohri N, Tomé WA, Méndez Romero A, Miften M, Ten Haken RK, Dawson LA, Grimm J, Yorke E, Jackson A. Local control following stereotactic body radiation therapy for liver tumors. Int J Radiat Oncol Biol Phys. [HyTEC issue, In Press]
Miften M, Vinogradskiy Y, Moiseenko V, Grimm J, Yorke E, Jackson A, Tomé WA, Ten Haken R, Ohri N, Méndez Romero A, Goodman KA, Marks LB, Kavanagh B, Dawson LA. Radiation dose-volume effects for liver SBRT. Int J Radiat Oncol Biol Phys. [HyTEC issue, In Press]
Puck TT, Marcus PI (1956) Action of x-rays on mammalian cells. J Exp Med 103(5):653–666
Borst GR, Ishikawa M, Nijkamp J, Hauptmann M, Shirato H, Bengua G, Onimaru R, de Josien BA, Lebesque JV, Sonke JJ (2010) Radiation pneumonitis after hypofractionated radiotherapy: evaluation of the LQ(L) model and different dose parameters. Int J Radiat Oncol Biol Phys 77(5):1596–1603
Fowler JF (2010) 21 years of biologically effective dose. Br J Radiol 83(991):554–568
Dale RG (1985) The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiol 58(690):515–528
Fowler JF, Stern BE (1958) Dose-rate factors in integral dose estimations [letter]. Br J Radiol 31:316
Fowler JF, Stern BE (1960) Dose-rate effects: some theoretical and practical considerations. Br J Radiol 33:389–395
Fowler JF (1989) The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 62(740):679–694
Hall EJ, Giacca AJ (2012) Radiobiology for the radiologist, 7th edn. Lippincott Williams & Wilkins, Philadelphia
Joiner M, Avd K (eds) (2009) Basic clinical radiobiology 4th Edition. Hodder Arnold, Great Britain
Brown JM, Brenner DJ, Carlson DJ (2013) Dose escalation, not “new biology,” can account for the efficacy of stereotactic body radiation therapy with non-small cell lung cancer. Int J Radiat Oncol Biol Phys 85(5):1159–1160
Brown JM, Carlson DJ, Brenner DJ (2014) The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys 88(2):254–262
Kehwar TS, Rathore RP, Supe SJ, Gupta MK (1995) Many component model and its parameters to fractionated irradiation. Strahlenther Onkol 171(10):573–580
Guerrero M, Li XA (2004) Extending the linear-quadratic model for large fraction doses pertinent to stereotactic radiotherapy. Phys Med Biol 49(20):4825–4835
Kirkpatrick JP, Marks LB (2004) Modeling killing and repopulation kinetics of subclinical cancer: direct calculations from clinical data. Int J Radiat Oncol Biol Phys 58:641–654
Carlone M, Wilkins D, Raaphorst P (2005) The modified linear-quadratic model of Guerrero and Li can be derived from a mechanistic basis and exhibits linear-quadratic-linear behaviour. Phys Med Biol. 50(10):L9–13; author reply L13–5.
Tai A, Erickson B, Khater KA, Li XA (2008) Estimate of radiobiologic parameters from clinical data for biologically based treatment planning for liver irradiation. Int J Radiat Oncol Biol Phys 70(3):900–907
Park C, Papiez L, Zhang S, Story M, Timmerman RD (2008) Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 70(3):847–852
Fowler JF (2008) Linear quadratics is alive and well: in regard to Park et al. (Int J Radiat Oncol Biol Phys 2008;70:847–852). Int J Radiat Oncol Biol Phys 72(3):957 author reply 958
Tomé WA (2009) Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy: in regard to Parks et al. (Int J Radiat Oncol Biol Phys 2008;72:1620–1621). Int J Radiat Oncol Biol Phys 73(4):1286
Guerrero M, Carlone M (2010) Mechanistic formulation of a lineal-quadratic-linear (LQL) model: split-dose experiments and exponentially decaying sources. Med Phys 37(8):4173–4181
Hanin LG, Zaider M (2010) Cell-survival probability at large doses: an alternative to the linear-quadratic model. Phys Med Biol 55:4687–4702
Kirkpatrick JP, Meyer JJ, Marks LB (2008) The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol 18:240–243
Brenner DJ (2008) The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin Radiat Oncol 18:234–239
Song CW, Cho LC, Yuan J, Dusenbery KE, Griffin RJ, Levitt SH (2013) Radiobiology of stereotactic body radiation therapy/stereotactic radiosurgery and the linear-quadratic model. Int J Radiat Oncol Biol Phys 87(1):18–19
Rao SS, Oh JH, Jackson A, Deasy JO (2014) Dose escalation, not “new biology,” can account for the efficacy of stereotactic body radiation therapy with non-small cell lung cancer. In regard to Brown et al. Int J Radiat Oncol Biol Phys 89(3):692–3
Song CW, Kim MS, Cho LC, Dusenbery K, Sperduto PW (2014) Radiobiological basis of SBRT and SRS. Int J Clin Oncol 19(4):570–578
Sperduto PW, Song CW, Kirkpatrick JP, Glatstein E (2015) A hypothesis: indirect cell death in the radiosurgery era. Int J Radiat Oncol Biol Phys 91(1):11–13
Song CW, Lee Y-J, Griffin RJ, Park I, Koonce NA, Hui S, Kim M-S, Dusenbery KE, Sperduto PW, Cho LC (2015) Indirect tumor cell death after high dose hypo-fractionated irradiation: implications for SBRT and SRS. Int J Radiat Oncol Biol Phys 93:166–172
Shuryak I, Carlson DJ, Brown JM, Brenner DJ (2015) High-dose and fractionation effects in stereotactic radiation therapy: analysis of tumor control data from 2965 patients. Radiother Oncol 115(3):327–334
Emami B, Woloschak G, Small W Jr (2015) Beyond the linear quadratic model: intraoperative radiotherapy and normal tissue tolerance. Transl Cancer Res 4(2):140–147
Leksell L (1971) Sterotaxic radiosurgery in trigeminal neuralgia. Acta Chir Scand 137(4):311–314
Barcia-Salorio JL, Broseta J, Hernandez G, Roldan P, Bordes V (1982) A new approach for direct CT localization in stereotaxis. Appl Neurophysiol 45(4–5):383–386
Betti OO, Derechinsky YE (1982) Irradiations stereotaxiques multifaisceaux. Neurochirurgie 28:55–56
Colombo F, Benedetti A, Pozza F, Avanzo RC, Marchetti C, Chierego G, Zanardo A (1985) External stereotactic irradiation by linear accelerator. Neurosurgery 16(2):154–160
Hartmann GH, Schlegel W, Sturm V, Kober B, Pastyr O, Lorenz WJ (1985) Cerebral radiation surgery using moving field irradiation at a linear accelerator facility. Int J Radiat Oncol Biol Phys 11(6):1185–1192
Lutz W, Winston KR, Maleki N (1988) A system for stereotactic radiosurgery with a linear accelerator. Int J Radiat Oncol Biol Phys 14(2):373–381
Friedman WA, Bova FJ (1989) The University of Florida radiosurgery system. Surg Neurol 32(5):334–342
Lax I, Blomgren H, Näslund I, Svanström R (1994) Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol 33(6):677–683
Adler JR (2009) Accuray, Inc. A neurosurgical business case. Cureus 1(9):e1. https://doi.org/10.7759/cureus.1
Suit H, Wette R (1966) Radiation dose fractionation and tumor control probability. Radiat Res 29(2):267–281
Burman C, Kutcher GJ, Emami B, Goitein M (1991) Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 21(1):123–135
Jackson A, Marks LB, Bentzen SM, Eisbruch A, Yorke ED, Ten Haken RK, Constine LS, Deasy JO (2010) The lessons of QUANTEC: recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int J Radiat Oncol Biol Phys 76(3 Suppl):S155–S160
Deasy JO, Bentzen SM, Jackson A, Ten Haken RK, Yorke ED, Constine LS, Sharma A, Marks LB (2010) Improving normal tissue complication probability models: the need to adopt a “data-pooling” culture. Int J Radiat Oncol Biol Phys 76(3 Suppl):S151–S154
Williamson JF, Das SK, Goodsitt MS, Deasy JO (2017) Introducing the medical physics dataset article. Med Phys 44(2):349–350
Grimm J, LaCouture T, Zhu Y, Xue J, Yeo I, Croce RJ (2011) Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys 12(2):267–292
Timmerman RD (2008) An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol 18:215–222
Kim DWN, Medin PM, Timmerman RD (2017) Emphasis on repair, not just avoidance of injury, facilitates prudent stereotactic ablative radiotherapy. Semin Radiat Oncol 27(4):378–392
Benedict S, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L, Purdie T, Sadagopan R, Schell MC, Salter B, Schlesinger DJ, Shiu AS, Solberg T, Song DY, Stieber V, Timmerman R, Tomé WA, Verellen D, Wang L, Yin FF (2010) Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 37(8):4078–4101
Bezhak A, Bradley J, Gaspar L, et al Seamless phase I/II study of stereotactic lung radiotherapy (SBRT) for early stage, centrally located, non-small cell lung cancer (NSCLC) in medically inoperable patients. Radiation Therapy Oncology Group 0813. Accessible at: https://www.rtog.org/ClinicalTrials/ProtocolTable.aspx. Accessed 8 Jun 2015
Videtic G, Singh A, Chang J, et al A randomized phase II study comparing 2 stereotactic body radiation therapy (SBRT) schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer. Radiation Therapy Oncology Group 0915. Accessible at: https://www.rtog.org/ClinicalTrials/ProtocolTable.aspx. Accessed 1 Nov 2012
Daly ME, Chen AM, Bucci MK, El-Sayed I, Xia P, Kaplan MJ, Eisele DW (2007) Intensity-modulated radiation therapy for malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys 67(1):151–157
Lee N, Garden A, Pfister D, et al. RTOG 0615 A phase II study of concurrent chemoradiotherapy using three-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiation therapy (IMRT) + bevacizumab (BV) for locally or regionally advanced nasopharyngeal cancer
National Cancer Institute. Common terminology criteria for adverse events v4.0. NIH publication # 09–7473. NCI, NIH, DHHS. May 29, 2009
Chapman JD, Nahum A (2015) Radiotherapy treatment planning, linear-quadratic radiobiology. CRC Press, Taylor & Francis Group LLC, Boca Raton
Kirkpatrick JP, van der Kogel AJ, Schultheiss TE (2010) Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys 76(3 Suppl):S42–S49
Grimm J, Shen C, Redmond KJ, Quon H, Kleinberg LR. Avoiding necrosis in radiosurgery of non-AVM intracranial tumors. ACRO 2017
Grimm J, Shen CJ, Redmond KJ, Sloan L, Hazell S, Seo Y, Chan L, Nikolaidis D, Moore J, Huang E, Quon H, Bettegowda C, Lim M, Kleinberg LR. Low Risk of Symptomatic Radionecrosis Following Stereotactic Radiosurgery for Multiple Brain Metastases, ASTRO 2017
Inoue HK, Seto K, Nozaki A, Torikai K, Suzuki Y, Saitoh JI, Noda SE, Nakano T (2013) Three-fraction CyberKnife radiotherapy for brain metastases in critical areas: referring to the risk evaluating radiation necrosis and the surrounding brain volumes circumscribed with a single dose equivalence of 14 Gy (V14). J Radiat Res 54(4):727–735
Inoue HK, Sato H, Seto KI, Torikai K, Suzuki Y, Saitoh JI, Noda SE, Nakano T (2014) Five-fraction CyberKnife radiotherapy for large brain metastases in critical areas: impact on the surrounding brain volumes circumscribed with a single dose equivalent of 14 Gy (V14) to avoid radiation necrosis. J Radiat Res 55(2):334–342
Fowler J, Yang J, Lamond J, Lanciano R, Feng J, Brady L (2010) A “Red Shell” concept of increased radiation damage hazard to normal tissues just outside the PTV target volume. Radiother Oncol 94(3):384
Yang J, Fowler JF, Lamond JP, Lanciano R, Feng J, Brady LW (2010) Red shell: defining a high-risk zone of normal tissue damage in stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 77(3):903–909
Yang J, Lamond J, Fowler J, Lanciano R, Feng J, Brady L (2013) Effect of fractionation in stereotactic body radiation therapy using the linear quadratic model. Int J Radiat Oncol Biol Phys 86(1):150–156
Lunsford LD (2009) Top 25 cited Gamma Knife articles in the Journal of Neurosurgery. J Neurosurg 111(3):1–2
Depp JG. Personal communication, founding president and CEO, Accuray Incorporated. Accessible via http://quantum.site.nfoservers.com/Joe/. Accessed 12 Aug 2017
Timmer FC, Hanssens PE, van Haren AE, Mulder JJ, Cremers CW, Beynon AJ, van Overbeeke JJ, Graamans K (2009) Gamma knife radiosurgery for vestibular schwannomas: results of hearing preservation in relation to the cochlear radiation dose. Laryngoscope 119(6):1076–1081
Gardner G, Robertson JH (1988) Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol, Rhinol, Laryngol 97:55–66
Schell MJ, McHaney VA, Green AA, Kun LE, Hayes FA, Horowitz M, Meyer WH (1989) Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. J Clin Oncol 7(6):754–760
Ross CJ, Katzov-Eckert H, Dubé MP, Brooks B, Rassekh SR, Barhdadi A, Feroz-Zada Y, Visscher H, Brown AM, Rieder MJ, Rogers PC, Phillips MS, Carleton BC, Hayden MR, CPNDS Consortium (2009) Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet 41(12):1345–1349
Brock PR, Maibach R, Childs M, Rajput K, Roebuck D, Sullivan MJ, Laithier V, Ronghe M, Dall’Igna P, Hiyama E, Brichard B, Skeen J, Mateos ME, Capra M, Rangaswami AA, Ansari M, Rechnitzer C, Veal GJ, Covezzoli A, Brugières L, Perilongo G, Czauderna P, Morland B, Neuwelt EA (2018) Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med 378(25):2376–2385
Ishikawa E, Sugimoto H, Hatano M, Nakanishi Y, Tsuji A, Endo K, Kondo S, Wakisaka N, Murono S, Ito M, Yoshizaki T (2015) Protective effects of sodium thiosulfate for cisplatin-mediated ototoxicity in patients with head and neck cancer. Acta Otolaryngol 135(9):919–924
Lawrence YR, Li XA, el Naqa I, Hahn CA, Marks LB, Merchant TE, Dicker AP (2010) Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys 76(3 Suppl):S20–S27
Rubin P (1967) Clinical oncology for medical students and physicians a multidisciplinary approach. Rochester, American Cancer Society
Jackson A, Yorke E, Rosenzweig K (2006) The atlas of complication incidence: a proposal for a new standard for reporting the results of radiotherapy protocols. Semin Radiat Oncol 16:260–268
Kimsey F, McKay J, Gefter J, Milano MT, Moiseenko V, Grimm J, Berg R (2016) Dose-response model for chest wall tolerance of stereotactic body radiation therapy. Semin Radiat Oncol 26(2):129–134
Hindo WA, DeTrana FA 3rd, Lee MS, Hendrickson FR (1970) Large dose increment irradiation in treatment of cerebral metastases. Cancer 26:138–141
Bijl HP, van Luijk P, Coppes RP, Schippers JM, Konings AW, van Der Kogel AJ (2005) Regional differences in radiosensitivity across the rat cervical spinal cord. Int J Radiat Oncol Biol Phys 61(2):543–551
Philippens ME, Pop LA, Visser AG, Peeters WJ, van der Kogel AJ (2009) Bath and shower effect in spinal cord: the effect of time interval. Int J Radiat Oncol Biol Phys 73(2):514–522
van Luijk P, Faber H, Schippers JM, Brandenburg S, Langendijk JA, Meertens H, Coppes RP (2009) Bath and shower effects in the rat parotid gland explain increased relative risk of parotid gland dysfunction after intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 74(4):1002–1005
Xue J, Goldman HW, Grimm J, LaCouture T, Chen Y, Hughes L, Yorke E (2012) Dose-volume effects on brainstem dose tolerance in radiosurgery. J Neurosurg 117(Suppl):189–196
Wang JZ, Huang Z, Lo SS, Yuh WT, Mayr NA (2010) A generalized linear-quadratic model for radiosurgery, stereotactic body radiation therapy, and high-dose rate brachytherapy. Sci Transl Med 2(39):39ra48
Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J (2010) Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys 76(3 Suppl):S28–S35
Mayo C, Yorke E, Merchant TE (2010) Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys 76(3 Suppl):S36–S41
Barendsen GW (1982) Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys 8(11):1981–1997
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was partially supported by a grant from Accuray.
Conflict of interest
Dr. Kleinberg has received research grants from Novocure, Arbor, Accuray, has performed consulting for Novocure, Accuray, and is on the advisory board for Novocure. Dr. Redmond has received research funding from Elekta AB and Accuray, as well as travel expenses and honorarium for speaking for Accuray. Dr. Grimm developed and holds intellectual property rights to the DVH Evaluator software tool which is an FDA-cleared product in commercial use, and which has been used for this analysis; and has received research grants from Novocure and Accuray. All other authors declare that they have no relevant conflict of interest.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent
Informed consent was not applicable due to the retrospective nature of brain dose tolerance study. All other data was from the published literature.
Additional information
In memory of Dr. Jack Fowler, DSc, PhD, MD (Hon), FIPEM, FInstP, FRCR, FACR, FASTRO, FAAPM
Rights and permissions
About this article
Cite this article
Xue, J., Emami, B., Grimm, J. et al. Clinical evidence for dose tolerance of the central nervous system in hypofractionated radiotherapy. J Radiat Oncol 7, 293–305 (2018). https://doi.org/10.1007/s13566-018-0367-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-018-0367-2