Abstract
Introduction
This study assessed the cost-effectiveness of secukinumab compared with other biologics (adalimumab, infliximab, ustekinumab, ixekizumab, guselkumab, and Yisaipu [etanercept biosimilar]) for moderate-to-severe plaque psoriasis from the Chinese healthcare system perspective.
Methods
A decision-tree (first year)/Markov model (subsequent years), with an annual cycle, was implemented over a lifetime horizon. The Psoriasis Area and Severity Index (PASI) response rate at week 16 was used for treatment response. Efficacy inputs were obtained from a mixed-treatment comparison conducted using data from randomized controlled trials. Other clinical inputs (adverse events, dropout, and mortality rates), utility weights, and costs were derived from published literature and local Chinese sources. Both costs and outcomes were discounted at 5% per annum. Model outcomes included quality-adjusted life years (QALYs) and incremental cost-effectiveness ratio (ICER). One-way and probabilistic sensitivity analyses were conducted to test the robustness of results.
Results
For patients with moderate-to-severe psoriasis, secukinumab generated the highest QALYs (12.334) against all comparators at a lifetime cost of ¥231,477. Secukinumab dominated (higher QALYs at lower costs) all other biologics except ixekizumab in this population. Compared with secukinumab, ixekizumab incurred slightly lower costs (¥228,320) but gained lesser QALYs (12.284). Thus, secukinumab was a cost-effective treatment than ixekizumab at a willingness-to-pay (WTP) threshold of ¥257,094 per QALY gained. In the one-way sensitivity analysis, base-case results were most sensitive to changes in the PASI response at 16 weeks and year 2+ dropout rates.
Conclusion
Secukinumab is the most cost-effective treatment option for patients with moderate-to-severe psoriasis compared with other commonly used biologics from the Chinese healthcare system perspective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
This study constitutes the first comprehensive economic evaluation of secukinumab compared with other commonly used biologics for moderate-to-severe plaque psoriasis in China. |
Patients receiving secukinumab generate the highest quality-adjusted life years (12.334) against all comparators at a lifetime cost of ¥231,477. |
Secukinumab provides the best economic value compared with other commonly used biologics for the treatment of moderate-to-severe plaque psoriasis in China. |
Introduction
Psoriasis is a chronic, immune-mediated inflammatory disease of the skin [1]. The worldwide prevalence rate of psoriasis is 0.5%, which varies widely from 0.1% in Southeast Asia to 1.9% in Western Europe [2]. In China, it affects 7.6 million individuals, resulting into age-standardized prevalence rate of 0.4% [2, 3]. Plaque psoriasis is the most common type of psoriasis, accounting for over 96.0% of all cases [4, 5]. On the basis of the body surface area (BSA) involvement, around 57.3% of patients have moderate-to-severe disease [4]. Psoriasis is associated with several comorbidities including hyperlipidemia, hypertension, diabetes, obesity, cardiovascular diseases, depression, and non-alcoholic fatty liver disease [1, 4, 6, 7]. Patients with psoriasis experience a significant functional, psychological, and social burden impacting their professional lives and leading to reduced quality of life (QoL) [8, 9]. In China, approximately 61.8% of patients with psoriasis report a severe or extremely severe impact on their QoL [10]. According to World Psoriasis Happiness Report 2018, Chinese people with self-reported psoriasis report the lowest average happiness levels (4 on a scale of 0–10) and more than 51.4% of them live in misery [11].
Treatments for psoriasis include topical agents, ultraviolet phototherapy, conventional systemic therapy (methotrexate and cyclosporine), retinoids, and biologics. The Chinese guidelines for the treatment of psoriasis recommend the use of biologics among patients with moderate-to-severe psoriasis who have not responded to, are intolerant of, or have contraindications to traditional systemic therapies [12,13,14]. Clinically used biologics in China include tumor necrosis factor alpha (TNFα) inhibitors (etanercept biosimilar, infliximab, and adalimumab), interleukin (IL)-12/IL-23 inhibitors (ustekinumab and guselkumab), and IL-17A inhibitors (secukinumab and ixekizumab) [14].
Patients with moderate-to-severe psoriasis usually require lifelong treatment [15]. Hence, psoriasis imposes a large economic burden on patients and their families [10, 16, 17]. In China, the total annual expenditure due to psoriasis accounts for approximately 20.0% of patients’ income and results into an annual hospitalization rate of 21.3%, 15.0 days of sick leave, and an unemployment rate of 37.0% [10]. Thus, it is important to assess the cost-effectiveness of current treatments to better allocate the finite healthcare resources.
Secukinumab is a fully human monoclonal antibody that selectively neutralizes IL-17A and has been approved for moderate-to-severe plaque psoriasis in China. It has demonstrated rapid onset of action and long-lasting efficacy with a favorable safety profile in the treatment of moderate-to-severe plaque psoriasis [18,19,20]. Several economic evaluations have been conducted in the USA, Canada, Japan, and Europe that assessed the cost-effectiveness of different biologics including secukinumab for the treatment of psoriasis [21,22,23,24,25,26,27,28,29,30,31,32]. However, studies evaluating the cost-effectiveness of secukinumab in the Chinese setting are scarce [33]. Therefore, this study aimed to assess the cost-effectiveness of secukinumab versus other commonly used biologics (adalimumab, infliximab, ustekinumab, ixekizumab, guselkumab, and Yisaipu [etanercept biosimilar]) for moderate-to-severe plaque psoriasis in China.
Methods
Patient Population and Interventions
The target patient population for the model was based on the pivotal phase 3 clinical trial of secukinumab (CAIN457A2318) [18]. The analysis included Chinese patients (aged ≥ 18 years) with moderate-to-severe chronic plaque psoriasis for at least 6 months who were inadequately controlled by topical agents, phototherapy, and/or conventional systemic therapy.
The following treatments and their respective dosage according to approved product label were considered for the cost-effectiveness analysis: secukinumab (150 mg for body weight < 60 kg and 300 mg for body weight ≥ 60 kg), adalimumab 40 mg, infliximab 5 mg/kg, ustekinumab 45 mg, ixekizumab 80 mg, guselkumab 100 mg, and Yisaipu 50 mg (Table 1).
Model Structure
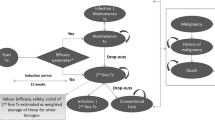
A decision-tree (first year)/Markov model (subsequent years), with an annual cycle, was implemented in Microsoft® Excel to compare secukinumab with commonly used biologics in China (Fig. 1a, b). The model structure was adapted from the previously published cost-effectiveness studies of secukinumab for psoriasis [23,24,25,26]. Patients entered the model at 39 years, based on the average age of patients in studies evaluating secukinumab in China [18, 34]. Treatment initiation was considered as the entry point for patients with a 16-week induction period. Efficacy assessment was conducted at weeks 4, 8, 12, and 16 using the Psoriasis Area and Severity Index (PASI).
On the basis of PASI scores, patients were assigned to three PASI-defined health states: PASI < 50 (non-responder), PASI 50–74 (partial responder), and PASI ≥ 75 (responder). Different PASI levels represented the corresponding percentage reduction in the PASI score from the baseline. The decision to continue patients on biologic treatment was assessed at 16 weeks, based on a response threshold of PASI ≥ 75. At week 16, patients with a PASI ≥ 75 response continued with the same biologic treatment. Non-responders (PASI < 50) and partial responders (PASI 50–74) to biologics discontinued active treatment and switched to standard of care at week 16 assessment. Standard of care treatment included methotrexate, cyclosporine, topical corticosteroids, and phototherapy [12,13,14]. Switching to a second-line biologic was not considered in the current model.
Given the chronic nature of psoriasis, patients with a PASI ≥ 75 response at week 52 continued the same biologic treatment and entered the long-term Markov in the “active treatment” health state until they dropout to “standard of care” or “death”. Patients who switched to standard of care treatment at any stage of model remained on it until death or the end of model time horizon. The model also considered a dropout rate for patients who were on active treatment between 16 and 52 weeks.
Model Assumptions
Non-responders (PASI < 50) to initial treatment were assumed to remain in that disease state with the standard of care until natural death. In patients with a sustained response at year 1, the PASI response state at week 16 was assumed to continue through week 52. Among patients who responded at week 16, but did not sustain their response at year 1, the PASI response state at week 16 was assumed to continue until they dropout at the midpoint between week 16 and 52. The dosing regimen for biologics was assumed to be in line with the approved label dose. No administration cost was assumed for subcutaneously (sc) administered treatment. For intravenous (iv) administration of infliximab, an additional visit cost was considered for each infusion.
Model Inputs
Efficacy Inputs
The PASI response rates at weeks 4, 8, 12, and 16 for all modeled interventions except guselkumab were obtained from a recently published mixed-treatment comparison, which compared the efficacy of secukinumab with other biologics in Chinese patients with moderate-to-severe plaque psoriasis (Table 2) [35]. For guselkumab, none of the identified studies reported data for the Chinese subpopulation at the time of analysis. Therefore, previously published network meta-analysis (NMA) by Pan et al. [36] was updated with a wider scope in January 2022 to identify the latest evidence on comparative efficacy of secukinumab and all other biologics for the treatment of moderate-to-severe plaque psoriasis (Table 2). Bayesian NMA method was used to combine evidence from the identified randomized controlled trials (RCTs). Details of the NMA methods and results are reported in the Supplementary Appendix A. For secukinumab, PASI response rate was assumed to be a weighted average of the response rate for patients receiving secukinumab 150 mg and those receiving 300 mg in the ratio of 32.3% and 67.7%, respectively. This ratio was derived from a real-world study evaluating the efficacy and safety of secukinumab treatment in Chinese patients with psoriasis. Study details are reported in the Supplementary Appendix B.
Dropout Rate Inputs
The dropout rate for the secukinumab arm was assumed to be a weighted average of the rate for secukinumab 150 mg (12.6%) and 300 mg (9.7%) arms in the ratio of 32.3% and 67.7%, respectively, based on data from a long-term phase 3 trial of secukinumab (ERASURE). For year 1, a dropout rate of 10.6% was estimated for the secukinumab arm [19]. For all other interventions, the year 1 dropout rate was assumed to be equivalent to that of the secukinumab arm. Beyond 1 year, a constant annual dropout rate of 20.0% was applied for all interventions representing a long-term adherence pattern to biologics based on published literature [37].
Adverse Events Inputs
Serious adverse events considered for analysis included non-melanoma skin cancer (NMSC), other malignancies, and severe infections (Table 3). Severe infections included sepsis, tuberculosis, pneumonia, skin and soft tissue infections, bone and joint infections, and urinary tract infections.
Mortality Inputs
The general annual mortality rates per 100,000 individuals were considered in the model, derived from the China Population and Employment Statistical Yearbook 2021 (Supplementary Table 5) [41].
Utility Inputs
Given the absence of preference-based health state utility estimates for Chinese patients with psoriasis, utility weights classified by PASI scores were derived using the Dermatology Life Quality Index (DLQI) data from a clinical trial of secukinumab evaluating patients with moderate-to-severe psoriasis in China (CAIN457A2318) [18]. An ordinal logistic regression method was implemented to map DLQI data to EQ-5D-3L-based utility estimates using the Chinese EQ-5D value set (Table 4) [42, 43]. Disutilities associated with methotrexate and cyclosporine, and the proportion of patients using each therapy are available in Table 4.
Cost and Resource Use Inputs
The model considered direct medical costs (without co-pay) which included drug acquisition costs, medical support costs (physician visits and monitoring), and adverse event costs (inpatient episode). All cost inputs were inflated to 2022 using the Chinese consumer price index (Table 5). The resource use pattern for interventions represented in the model is available in Supplementary Table 6.
Base-Case Analysis
The base-case analysis assessed the cost-effectiveness of secukinumab compared with other biologics from the Chinese healthcare system perspective. The primary effectiveness outcome was quality-adjusted life years (QALYs). The incremental cost-effectiveness ratio (ICER) was calculated and a treatment with higher QALYs at lower costs against a comparator was considered “dominant.”
The base-case analysis was conducted over a lifetime horizon to comprehensively evaluate all relevant costs and health effects for this chronic condition. An annual discount rate of 5% was applied to both costs and outcomes [52]. A willingness-to-pay (WTP) threshold for China was considered to be three times the gross domestic product per capita in 2022 and set at ¥257,094/QALY [53].
Sensitivity Analyses
One-way sensitivity analysis and probabilistic sensitivity analysis (PSA) were performed to assess the robustness of the study findings. In the one-way sensitivity analysis, the model input parameters such as PASI response, dropout rate, adverse events, utility inputs, discount rate, and costs were varied to identify sensitive parameters with the greatest effect on the model results. A list of the parameters and their ranges is provided in the Supplementary Table 7.
In the PSA, the uncertainty around the results was determined by running the model 10,000 times with a certain distribution of each input parameter (response rate, dropout rate, costs, and utility weights). The analysis results were presented using cost-effectiveness acceptability curves estimated using the net monetary benefit (NMB) statistic for a range of WTP thresholds for each treatment. Scenario analyses were performed using different proportions for patients receiving secukinumab 150 mg and 300 mg (44.3% vs 55.7%, 59.5% vs 40.5%, and 70.6% vs 29.4%, respectively) based on real-world studies conducted in China. Details are provided in Supplementary Table 8. Another scenario analysis was conducted using alternative prices for adalimumab and infliximab based on the average retail price weighted according to their market share in China (Supplementary Table 9).
Compliance with Ethics Guidelines
This article is based on mathematical modeling with inputs informed primarily by previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Base-Case Results
Among patients with moderate-to-severe plaque psoriasis, secukinumab generated the highest QALYs (12.334) at a lifetime cost of ¥231,477. The iv administered infliximab achieved the second highest QALYs (12.330) followed by ixekizumab, guselkumab, adalimumab, ustekinumab, and Yisaipu. The total costs and QALYs for all the interventions are presented in Table 6.
With the highest number of QALYs at lower costs, secukinumab dominated all other biologics except ixekizumab in this population. Although patients receiving ixekizumab incurred marginally lower costs (¥228,320) than those receiving secukinumab, they gained fewer QALYs (12.284). Therefore, secukinumab was a cost-effective option compared with ixekizumab at a WTP threshold of ¥257,094 per QALY gained (Table 6).
Sensitivity Analyses
In the one-way sensitivity analysis, the PASI response at 16 weeks and year 2+ dropout rates were found to be the most sensitive parameters affecting the model results for all treatment comparisons. The detailed results are shown using the tornado diagrams in Supplementary Fig. 4. The PSA results demonstrated that secukinumab was likely to provide the highest NMB in 76% of the simulations (Supplementary Table 10). Furthermore, the cost-effectiveness acceptability curve demonstrated that secukinumab had the highest probability of being cost-effective compared to other biologics at a WTP of ¥85,000 and above per QALY gained (Fig. 2).
In the scenario analyses with higher proportion of patients receiving secukinumab 150 mg versus 300 mg, secukinumab was the most cost-effective treatment compared with all other biologics except infliximab. Patients receiving infliximab gained slightly higher QALYs but at higher costs than those receiving secukinumab in all three scenarios. This resulted in an ICER of ¥23,933,817/QALY (proportion of patients receiving secukinumab 150 mg, 44.3%), ¥7,659,166/QALY (proportion of patients receiving secukinumab 150 mg, 59.5%), and ¥5,095,731/QALY (proportion of patients receiving secukinumab 150 mg, 70.6%), respectively, for infliximab compared with secukinumab. Thus, infliximab was not a cost-effective option compared with secukinumab at a WTP threshold of ¥257,094 per QALY gained. Similar to base-case analysis, secukinumab also dominated adalimumab and infliximab in the scenario analyses conducted using the alterative drug prices for adalimumab and infliximab, respectively. The results for scenario analyses are presented in Supplementary Table 11.
Discussion
The present study assessed the cost-effectiveness of secukinumab compared with other commonly used biologics for moderate-to-severe plaque psoriasis in China from the healthcare system perspective. To our knowledge, this is the first comprehensive economic evaluation comparing the costs and benefits (QALYs) associated with secukinumab versus other commonly used biologics in Chinese settings.
We found that secukinumab was a cost-effective treatment option compared with adalimumab, infliximab, ustekinumab, ixekizumab, guselkumab, and Yisaipu among patients with moderate-to-severe psoriasis over a lifetime horizon. The results of base-case analysis were most sensitive to changes in the PASI response at 16 weeks and year 2+ dropout rates for all treatment comparisons. The alternative scenario analyses also provided results similar to base-case analysis confirming the cost-effectiveness of secukinumab.
Our study results were aligned with similar evaluations conducted in other countries where secukinumab was a cost-effective option compared with adalimumab, etanercept, infliximab, and ustekinumab [24,25,26,27,28,29]. From the Japanese healthcare system perspective, secukinumab 300 mg dominated infliximab and ustekinumab, providing the highest QALYs at a lower cost in psoriasis treatment over a 5-year time horizon. The ICER for secukinumab compared with adalimumab was slightly higher than a WTP threshold of JP¥8,000,000 per QALY gained [24]. In the Italian National Health System (NHS) settings, secukinumab 300 mg was a cost-effective option against ustekinumab (dominant), adalimumab, etanercept, infliximab, and standard of care for the treatment of plaque psoriasis for over 10 years [25]. Similar findings were reported in a Canadian study evaluating the cost-effectiveness of secukinumab versus other biologics for plaque psoriasis over a 10-year time horizon. In this study, etanercept was strongly dominated, whereas adalimumab, ustekinumab, and secukinumab 150 mg were weakly dominated by secukinumab 300 mg. The ICER for infliximab versus secukinumab 300 mg was very high ($1,039,403 per QALY gained) [26]. In Germany, secukinumab 300 mg as the first-line treatment of moderate-to-severe psoriasis was the most cost-effective option that demonstrated the lowest cost per PASI 90 responder over 16 weeks as well as 52 weeks compared with adalimumab, etanercept, infliximab, and ustekinumab [27].
In contrast to our study findings, two studies reported modest cost savings and QALY gain with ixekizumab versus secukinumab in the treatment of moderate-to-severe psoriasis [30, 31]. In the UK, the cost-effectiveness of sequential biologics containing first-line ixekizumab versus first-line secukinumab (followed by ustekinumab, infliximab, and best supportive care) was assessed in patients with moderate-to-severe plaque psoriasis. Treatment with ixekizumab was associated with a marginal gain of 0.03 QALYs and cost savings of £898 compared with secukinumab over a lifetime horizon [30]. Similar results were obtained in another study evaluating ixekizumab versus secukinumab for moderate-to-severe plaque psoriasis in the Spanish NHS setting. Ixekizumab provided an additional 0.04 QALYs and potential savings of €1951 compared with secukinumab over a lifetime horizon [31]. One limitation of these analyses was exclusion of costs associated with serious adverse events requiring hospitalization [30, 31]. In the cost per responder analysis evaluating the guselkumab for the treatment of moderate-to-severe psoriasis in Germany, guselkumab had a lower cost per PASI 90 responder compared with adalimumab, apremilast, etanercept, infliximab, ixekizumab, secukinumab, tildrakizumab, and ustekinumab over a 1-year time horizon [32].
In China, one real-world study estimated the cost and effectiveness of adalimumab and secukinumab treatment for moderate-to-severe plaque psoriasis over 12 weeks. The cost per PASI 75 responder in the adalimumab group (¥17,581) was lower than that in the secukinumab group (¥46,332). This difference was attributed to the higher drug price of secukinumab (¥2998) than adalimumab (¥1290) during year 2019–2020 when only adalimumab was included in the national reimbursement drug list (NRDL) [33]. This study considered only the drug acquisition costs over 12 weeks, whereas in our analysis, different medical costs were accounted for the total cost estimation. In addition, our analysis used QALYs, the most preferred outcome measure for economic evaluation.
Various factors contributed to the strength of this analysis. Based on approved product label and common clinical practice in China, the current evaluation considered the weight-based dosing for secukinumab. In the absence of head-to-head RCTs, the comparative clinical efficacy data for different biologics were derived from an NMA, conducted using the Bayesian technique. Inclusion of costs for drug acquisition, medical support, and adverse events in the current analysis indicated the true economic burden of psoriasis on the Chinese healthcare system. To better reflect the preference of Chinese patients, the DLQI data was used to derive the EQ-5D-based utility estimates. A lifetime horizon was considered to account for the chronic course of the disease. Finally, the robustness of the model results was confirmed using both one-way and probabilistic sensitivity analyses.
Nonetheless, this model-based analysis has certain limitations. The model used short-term efficacy data to project lifetime efficacy, derived from the NMA which was restricted to week 16 because of crossover of treatment arms beyond week 12 or week 16. The other limitation was treatment sequencing, as the present analysis was restricted to first-line biologic treatment only. In clinical practice, patients who do not respond to first-line biologic can switch to another biologic agent. However, efficacy inputs for treatment sequencing are not readily available in the literature. Thus, results may be sensitive to assumptions about the choice and efficacy of subsequent treatments. Drug costs were estimated using the published list prices; therefore, this analysis did not include any confidential discounts. Dropout rates from the secukinumab trials were used to calculate the QALYs for all other biologics. Finally, indirect costs were not considered as the analysis was performed from a healthcare system perspective.
Conclusions
This cost-effectiveness analysis demonstrated that secukinumab is the most cost-effective treatment option compared with other commonly used biologics (adalimumab, infliximab, ustekinumab, ixekizumab, guselkumab, and Yisaipu) for moderate-to-severe plaque psoriasis in China over a lifetime horizon.
Data Availability
All data generated or analyzed during this study are included in this published article or as supplementary information files.
References
Boehncke WH, Schon MP. Psoriasis. Lancet. 2015;386(9997):983–94. https://doi.org/10.1016/S0140-6736(14)61909-7.
Damiani G, Bragazzi NL, Karimkhani Aksut C, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med (Lausanne). 2021;8:743180. https://doi.org/10.3389/fmed.2021.743180.
Ding X, Wang T, Shen Y, et al. Prevalence of psoriasis in China: a population-based study in six cities. Eur J Dermatol. 2012;22(5):663–7. https://doi.org/10.1684/ejd.2012.1802.
Chen K, Wang G, Jin H, et al. Clinic characteristics of psoriasis in China: a nationwide survey in over 12000 patients. Oncotarget. 2017;8(28):46381–9. https://doi.org/10.18632/oncotarget.18453.
Pan R, Zhang J. Epidemiology and treatment of psoriasis: a Chinese perspective. Psoriasis (Auckl). 2014;4:37–47. https://doi.org/10.2147/PTT.S51717.
Bu J, Ding R, Zhou L, et al. Epidemiology of psoriasis and comorbid diseases: a narrative review. Front Immunol. 2022;13:880201. https://doi.org/10.3389/fimmu.2022.880201.
Gisondi P, Bellinato F, Girolomoni G, et al. Pathogenesis of chronic plaque psoriasis and its intersection with cardio-metabolic comorbidities. Front Pharmacol. 2020;11:117. https://doi.org/10.3389/fphar.2020.00117.
Pariser D, Schenkel B, Carter C, et al. A multicenter, non-interventional study to evaluate patient-reported experiences of living with psoriasis. J Dermatolog Treat. 2016;27(1):19–26. https://doi.org/10.3109/09546634.2015.1044492.
Zhong H, Yang H, Mao Z, et al. Impact of moderate-to-severe psoriasis on quality of life in China: a qualitative study. Health Qual Life Outcomes. 2021;19(1):271. https://doi.org/10.1186/s12955-021-01902-w.
Chen X, Zheng L, Zhang H, et al. Disease burden and quality of life in patients with psoriasis: an internet-based questionnaire. Chin J Dermatol. 2019;52(11):791–5. https://doi.org/10.35541/cjd.20190247.
Leo Innovation Lab and The Happiness Research Institute. World Psoriasis Happiness Report 2018 [Internet]. Leo Innovation Lab and The Happiness Research Institute, Copenhagen, Denmark. https://ifpa-pso.com/resources-tools/world-psoriasis-happiness-report-2018. Accessed 17 Feb 2023.
Committee of Psoriasis, Dermatology Branch, Chinese Medical Association. Guidelines for the diagnosis and treatment of psoriasis in China: 2019 concise edition#. Int J Dermatol Venereol. 2020;3(1):14–26. https://doi.org/10.1097/JD9.0000000000000074.
Committee on Psoriasis, Chinese Society of Dermatology. Guideline for the diagnosis and treatment of psoriasis in China (2018 complete edition). Chin J Dermatol. 2019;52(10):667–710. https://doi.org/10.35541/cjd.20190847.
Chinese Society of Dermatology, China Dermatologist Association, Dermatology & Venereology Specialized Committee of Chinese Association of Integrative Medicine. Guidelines for the treatment of psoriasis with biologic agents in China. Chin J Dermatol. 2021;54(12):1033–47. https://doi.org/10.35541/cjd.20210643.
Rendon A, Schakel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. https://doi.org/10.3390/ijms20061475.
Al Sawah S, Foster SA, Goldblum OM, et al. Healthcare costs in psoriasis and psoriasis sub-groups over time following psoriasis diagnosis. J Med Econ. 2017;20(9):982–90. https://doi.org/10.1080/13696998.2017.1345749.
Schaefer CP, Cappelleri JC, Cheng R, et al. Health care resource use, productivity, and costs among patients with moderate to severe plaque psoriasis in the United States. J Am Acad Dermatol. 2015;73(4):585–93.e3. https://doi.org/10.1016/j.jaad.2015.06.049.
Cai L, Zhang JZ, Yao X, et al. Secukinumab demonstrates high efficacy and a favorable safety profile over 52 weeks in Chinese patients with moderate to severe plaque psoriasis. Chin Med J (Engl). 2020;133(22):2665–73. https://doi.org/10.1097/CM9.0000000000001163.
Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis-results of two phase 3 trials. N Engl J Med. 2014;371(4):326–38. https://doi.org/10.1056/NEJMoa1314258.
Thaci D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–9. https://doi.org/10.1016/j.jaad.2015.05.013.
Gutknecht M, Krensel M, Augustin M. Health economic analyses of psoriasis management: a systematic literature search. Arch Dermatol Res. 2016;308(9):601–16. https://doi.org/10.1007/s00403-016-1673-4.
Zhang W, Islam N, Ma C, et al. Systematic review of cost-effectiveness analyses of treatments for psoriasis. Pharmacoeconomics. 2015;33(4):327–40. https://doi.org/10.1007/s40273-014-0244-9.
National Institute for Health and Care Excellence (NICE). Secukinumab for treating moderate to severe plaque psoriasis, Technology appraisal guidance [TA350] [Internet]. National Institute for Health and Care Excellence (NICE), UK. 2015. https://www.nice.org.uk/guidance/ta350. Accessed 17 Feb 2023.
Igarashi A, Igarashi A, Graham CN, et al. Evaluating the cost-effectiveness of secukinumab in moderate-to-severe psoriasis: a Japanese perspective. J Med Econ. 2019;22:7–15. https://doi.org/10.1080/13696998.2018.1532905.
D’Ausilio A, Aiello A, Daniel F, et al. PSS50 - A cost-effectiveness analysis of secukinumab 300 mg vs current therapies for the treatment of moderate to severe plaque psoriasis in Italy. Value Health. 2015;18(7):A424. https://doi.org/10.1016/j.jval.2015.09.574.
Lee A, Gregory V, Gu Q, et al. PSS19 - Cost-effectiveness of secukinumab compared to current treatments for the treatment of moderate to severe plaque psoriasis in Canada. Value Health. 2015;18(3):A182. https://doi.org/10.1016/j.jval.2015.03.1051.
Augustin M, McBride D, Gilloteau I, et al. Cost-effectiveness of secukinumab as first biologic treatment, compared with other biologics, for moderate to severe psoriasis in Germany. J Eur Acad Dermatol Venereol. 2018;32(12):2191–9. https://doi.org/10.1111/jdv.15047.
Klimes J, Mollon P, Graham C, et al. PSS51 - Cost-effectiveness analysis of secukinumab compared to ustekinumab in the treatment of moderate to severe plaque psoriasis in the Czech Republic. Value Health. 2015;18(7):A424. https://doi.org/10.1016/j.jval.2015.09.575.
Costa-Scharplatz M, Lang A, Gustavsson A, et al. PSS42 - Cost-effectiveness of secukinumab compared to ustekinumab In patients with psoriasis from a Swedish health care perspective. Value Health. 2015;18(7):A422. https://doi.org/10.1016/j.jval.2015.09.566.
Johansson EC, Hartz S, Kiri SH, et al. Cost-effectiveness analysis of sequential biologic therapy with ixekizumab versus secukinumab as first-line treatment of moderate-to-severe psoriasis in the UK. J Med Econ. 2018;21(8):810–20. https://doi.org/10.1080/13696998.2018.1474747.
Johansson E, Nunez M, Svedbom A, et al. Cost effectiveness of ixekizumab versus secukinumab in the treatment of moderate-to-severe plaque psoriasis in Spain. Clinicoecon Outcomes Res. 2018;10:747–59. https://doi.org/10.2147/CEOR.S167727.
Augustin M, Wirth D, Mahlich J, et al. Cost per responder analysis of guselkumab versus targeted therapies in the treatment of moderate to severe plaque psoriasis in Germany. J Dermatolog Treat. 2022;33(2):976–82. https://doi.org/10.1080/09546634.2020.1793891.
Li G, Gu Y, Zou Q, et al. Efficacy, safety, and pharmacoeconomic analysis of adalimumab and secukinumab for moderate-to-severe plaque psoriasis: a single-center, real-world study. Dermatol Ther (Heidelb). 2022;12(9):2105–15. https://doi.org/10.1007/s13555-022-00787-x.
Zhao Y, Cai L, Liu XY, et al. Efficacy and safety of secukinumab in Chinese patients with moderate-to-severe plaque psoriasis: a real-life cohort study. Chin Med J (Engl). 2021;134(11):1324–8. https://doi.org/10.1097/CM9.0000000000001510.
Yu C, Zhang J. A network meta-analysis of the efficacy of biologics in the treatment of Chinese patients with moderate to severe psoriasis. Atopic Dermatitis and Immune Skin Disease Summit Forum. Beijing: Peking University; 2022.
Pan R, Wang X, Shu M, et al. Comparative efficacy of secukinumab against adalimumab and infliximab in patients with moderate-to-severe plaque psoriasis. Chin Med J (Engl). 2022;135(1):11–9. https://doi.org/10.1097/CM9.0000000000001817.
Woolacott N, Hawkins N, Mason A, et al. Etanercept and efalizumab for the treatment of psoriasis: a systematic review. Health Technol Assess. 2006;10(46):1–233, i–iv. https://doi.org/10.3310/hta10460.
Dixon WG, Watson K, Lunt M, et al. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54(8):2368–76. https://doi.org/10.1002/art.21978.
Armstrong A, Paul C, Puig L, et al. Safety of ixekizumab treatment for up to 5 years in adult patients with moderate-to-severe psoriasis: results from greater than 17,000 ptient-years of exposure. Dermatol Ther (Heidelb). 2020;10(1):133–50. https://doi.org/10.1007/s13555-019-00340-3.
Blauvelt A, Papp KA, Griffiths CE, et al. Efficacy and safety of guselkumab, an anti-interleukin-23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double-blinded, placebo- and active comparator-controlled VOYAGE 1 trial. J Am Acad Dermatol. 2017;76(3):405–17. https://doi.org/10.1016/j.jaad.2016.11.041.
National Bureau of Statistics of China. China Population and Employment Statistical Yearbook-2021 [Internet]. National Bureau of Statistics of China, China Statistics Press, Beijing. 2021. https://www.yearbookchina.com/navibooklist-n3022013208-1.html. Accessed 22 Feb 2023.
Ali FM, Kay R, Finlay AY, et al. Mapping of the DLQI scores to EQ-5D utility values using ordinal logistic regression. Qual Life Res. 2017;26(11):3025–34. https://doi.org/10.1007/s11136-017-1607-4.
Liu GG, Wu H, Li M, et al. Chinese time trade-off values for EQ-5D health states. Value Health. 2014;17(5):597–604. https://doi.org/10.1016/j.jval.2014.05.007.
Diamantopoulos A, Finckh A, Huizinga T, et al. Tocilizumab in the treatment of rheumatoid arthritis: a cost-effectiveness analysis in the UK. Pharmacoeconomics. 2014;32(8):775–87. https://doi.org/10.1007/s40273-014-0165-7.
Yaozhi. Drug maximum retail price (yuan) [Internet]. Yaozhi, China. 2022. https://db.yaozh.com/yaopinjiage. Accessed 22 Feb 2023.
Zhu C, Chen S, Zhu Y, et al. A retrospective study of hospitalization costs, length of hospital stay and mortality of hospital-acquired sepsis. Mod Hosp. 2018;18(11):1598–601. https://doi.org/10.3969/j.issn.1671-332X.2018.11.012.
Jin W, Zhang Y, Xu Z. Prediction of malignant tumor hospitalization, hospitalization cost and average hospitalization time based on ARIMA product seasonal model. Mod Hosp. 2019;19(3):383–9. https://doi.org/10.3969/j.issn.1671-332X.2019.03.021.
Chen L, Cheng L, Zhao X. ARIMA product season model for predicting number of inpatient and hospitalized expense of malignant tumor. China Health Stat. 2017;34(4):554–7.
Du Y, Nan J, Xie H, et al. Cost and effects incurred during quarantined inpatient treatment of smear and culture positive pulmonary tuberculosis. Chin J Antituberc. 2016;38(11):1000–2. https://doi.org/10.3969/j.issn.1000-6621.2016.11.021.
Xie Y, Han J, Yu W, et al. Analysis on the structure of hospitalization expenses of pulmonary tuberculosis patients in Tianjin from 2008 to 2017. Mod Prev Med. 2019;46(10):1820–4.
Lu X, Wang J, Bai L. Investigation and analysis of current status of operation of infection control departments of 201 hospitals. Chin J Hosp Infect. 2017;27(14):3339–42.
Xie Z, Li H. Discussion on discount rate in pharmacoeconomic evaluation for China. Chin Health Econ. 2019;38(5):74–7. https://caod.oriprobe.com/articles/56433534/Discussion_on_Discount_Rate_in_Pharmacoeconomic_Evaluation_for_China.htm. Accessed 3 Mar 2023.
National Bureau of Statistics of China. Statistical Bulletin of the People's Republic of China on the 2022 National Economic and Social Development [Internet]. China Statistics Press, Beijing. 2023. http://www.stats.gov.cn/english/PressRelease/202302/t20230227_1918979.html. Accessed 3 Mar 2023.
Acknowledgements
Medial Writing and Editorial Assistance
Amit Pagada, co-author and an employee of Novartis, provided the editorial assistance in the preparation of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Funding
This research and the rapid service fee was sponsored by Beijing Novartis Pharma Co., Ltd., Beijing, China.
Author information
Authors and Affiliations
Contributions
Jinsui Zhang and Zemin Xia were responsible for data interpretation and analysis. Material preparation and data collection were performed by Wanjie Guo, Xiaoxiao Ren and Fang Liu. Gargi Ratnaparkhi, Amit Pagada and Subhashini Subramanian conducted the literature review and network meta-analysis. Min Hu took charge of a study conception and design and contributed to interpretation and revision along with Wen Chen. The first draft of the manuscript was written by Jinsui Zhang and all authors commented on previous versions of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Jinsui Zhang, Zemin Xia, Min Hu and Wen Chen declare that they have no conflicts of interest. Wanjie Guo, Xiaoxiao Ren and Fang Liu are employees of Novartis Pharmaceuticals, Beijing, China. Gargi Ratnaparkhi, Amit Pagada and Subhashini Subramanian are employees of Novartis Healthcare Pvt. Ltd., Hyderabad, India.
Ethical Approval
This article is based on mathematical modeling with inputs informed primarily by previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhang, J., Xia, Z., Guo, W. et al. Cost-Effectiveness of Secukinumab Versus Other Biologics in the Treatment of Moderate-to-Severe Plaque Psoriasis: The Chinese Healthcare System Perspective. Dermatol Ther (Heidelb) 13, 2681–2696 (2023). https://doi.org/10.1007/s13555-023-01041-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-01041-8