Abstract
Background
Gestational diabetes mellitus (GDM) initiates when a woman’s pancreas could not act appropriately to bypass the diabetogenic condition during pregnancy. It is increasing across the world, including Bangladesh. Triglycerides (TG) and high-density lipoprotein-cholesterol (HDLC) are strongly connected with insulin resistance in pregnant women.
Objectives
Observation of the role of lipid profiles and TG/HDL cholesterol ratio associated with fasting glucose in GDM subjects.
Methods
In this experiment, a total of 232 individual subjects consisting of 132 GDM-positive and 100 GDM-negative pregnant women were examined and observed from 24 to 28 weeks of their pregnancy period. For this study, we had collected blood samples from selected women before and after breakfast and analyzed blood glucose level, triglyceride cholesterol level, HDL, low-density lipoprotein (LDL), and TG/HDL ratio.
Results
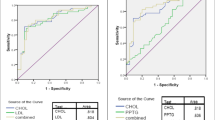
TG and LDL-cholesterol were significantly higher (p < 0.001) in GDM individuals (220.95 ± 67.4 and 149.54 ± 32.4, respectively) than those of the non-GDM (160.98 ± 59.67 and 129.18 ± 34.18, respectively). On the contrary, HDL-cholesterol level was comparatively lower in GDM-positive women than non-GDM subjects. In this case, the optimum cut-off point was 3.8 for the TG/HDL-C ratio with 62% sensitivity and 78% specificity by oral glucose tolerance test (OGTT).
Conclusion
Significantly (p < 0.001) higher TG/HDL ratio was found in GDM women compared with those in non‐GDM. TG/HDL ratios are independently associated with the risks of GDM, which might be a good marker in predicting GDM risk.
Similar content being viewed by others
Data availability
The dataset will be available upon request unless there are legal or ethical reasons for not doing so.
Code availability
Not applicable.
References
Bharathi KR, Vijayalakshmi S, Shrunga RP. A study of lipid parameters among GDM and non GDM pregnant women: a hospital based study. Int J Reprod Contraception Obstet Gynecol. 2017;6:5488.
Van Assche FA, Aerts L, Holemans K. Metabolic alterations in adulthood after intrauterine development in mothers with mild diabetes. Diabetes. 1991;40:106–8.
Van Assche FA, Aerts L, Holemans K. Maternal diabetes and the effect for the offspring. Verh K Acad Geneeskd Belg. 1992;54.
Rosenn B, Tsang RC. The effects of maternal diabetes on the fetus and neonate. Ann Clin Lab Sci. 1991;21:153–70.
American College of Obstetricians and Gynecologists. Assessment of risk factors for preterm birth. Clinical management guidelines for obstetrician-gynecologists. ACOG Practice Bulletin. 2001; 31.
Mohiuddin AK. Diabetes fact: Bangladesh perspective. Int J Diabetes Res. 2019;2:14–20.
American Diabetes Association. Gestational diabetes mellitus (Position Statement). Diabetes Care. 2004;27(Suppl. 1):S88-90.
Cheung NW, Wasmer G, Al-Ali J. Risk factors for gestational diabetes among Asian women. Diabetes Care. 2001;24:955–6.
Wang Y, Zhao X, Zhao H, Ding H, Tan J, Chen J, et al. Risks for gestational diabetes mellitus and pregnancy-induced hypertension are increased in polycystic ovary syndrome. Biomed Res Int. 2013;2013.
Monir N, Zeba Z, Rahman A. Comparison of knowledge of women with gestational diabetes mellitus and healthy pregnant women attending at hospital in Bangladesh. J Sci Found. 2018;16:20–6.
Li G, Kong L, Zhang L, Fan L, Su Y, Rose JC, et al. Early pregnancy maternal lipid profiles and the risk of gestational diabetes mellitus stratified for body mass index. Reprod Sci. 2015;22:712–7.
Zhu WW, Yang HX, Wei YM, Yan J, Wang ZL, Li XL, Wu HR, Li N, Zhang MH, Liu XH, Zhang H. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in China. Diabetes Care. 2013;36:586–90.
Nolan CJ, Riley SF, Sheedy MT, Walstab JE, Beischer NA. Maternal serum triglyceride, glucose tolerance, and neonatal birth weight ratio in pregnancy: a study within a racially heterogeneous population. Diabetes Care. 1995;18:1550–6.
Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768–73.
Trajman A, Luiz RR. McNemar. χ2 test revisited: comparing sensitivity and specificity of diagnostic examinations. Scand J Clin Lab Invest. 2008;68:77–80. https://doi.org/10.1080/00365510701666031.
Monteiro LJ, Norman JE, Rice GE, Illanes SE. Fetal programming and gestational diabetes mellitus. Placenta. 2016;48:S54-60.
Dorner G, Plagemann A, Reinagel H. Familial diabetes aggregation in type I 532 diabetics: gestational diabetes an apparent risk factor for increased diabetes 533 susceptibility in the offspring. Exp Clin Endocrinol. 1987;89:84–90.
Bartha JL, Comino-Delgado R, Martinez-Del-Fresno P, Fernandez-Barrios M, Bethencourt I, Moreno-Corral L. Insulin-sensitivity index and carbohydrate and lipid metabolism in gestational diabetes. J Reprod Med Obstet Gynecol. 2000;45:185–9.
Clark CM, Qiu C, Amerman B, Porter B, Fineberg N, Aldasouqi S, Golichowski A. Gestational diabetes: should it be added to the syndrome of insulin resistance? Diabetes Care. 1997;20:867–71.
Couch SC, Philipson EH, Bendel RB, Wijendran V, Lammi-Keefe CJ. Maternal and cord plasma lipid and lipoprotein concentrations in women with and without gestational diabetes mellitus: predictors of birth weight? J Reprod Med Obstet Gynecol. 1998;43:816–22.
Barat S, Ghanbarpour A, Bouzari Z, Batebi Z. Triglyceride to HDL cholesterol ratio and risk for gestational diabetes and birth of a large-for-gestational-age newborn. Casp J Intern Med. 2018;9:368–75.
Russell MA, Carpenter MW, Coustan DR. Screening and diagnosis of gestational diabetes mellitus. Clin Obstet Gynecol. 2007;50:949–58.
Wang J, Li Z, Lin L. Maternal lipid profiles in women with and without gestational diabetes mellitus. Medicine (Baltimore). 2019;98:e15320.
Donovan BM, Spracklen CN, Schweizer ML, Ryckman KK, Saftlas AF. Intimate partner violence during pregnancy and the risk for adverse infant outcomes: a systematic review and meta-analysis. BJOG An Int J Obstet Gynaecol. 2016;123:1289–99.
Meshkini M, Alaei-Shahmiri F, Mamotte C, Earnest J. Ethnic variation in lipid profile and its associations with body composition and diet: differences between Iranians, Indians and Caucasians living in Australia. J Immigr Minor Heal. 2017;19:67–73.
Feingold K, Brinton EA, Grunfeld C. The effect of endocrine disorders on lipids and lipoproteins. Endotext. 2020.
Sikaris KA. The clinical biochemistry of obesity. Clin Biochem Rev. 2004;25:165–81.
Bahrami H, Sadatsafavi M, Pourshams A, Kamangar F, Nouraei M, Semnani S, et al. Obesity and hypertension in an Iranian cohort study; Iranian women experience higher rates of obesity and hypertension than American women. BMC Public Health. 2006;6.
Lakshmi AS, Lakshmanan A, Ganesh Kumar P, Saravanan A. Effect of intensity of cigarette smoking on haematological and lipid parameters. J Clin Diagnostic Res. 2014;8.
Rashan MA, Dawood OT, Razzaq HAA, Hassali MA. The impact of cigarette smoking on lipid profile among Iraqi smokers. Int J Collab Res Intern Med Public Heal. 2016;8.
Halimi L, Haghdoost AA, Alizadeh SM. Prevalence of cigarette smoking among Iranian women: a systematic review and meta-analysis. Med J Islam Repub Iran. 2013;27:132–40.
Mansouri M, Sadeghi O, Roshanfekr P, Sharifi F, Varmaghani M, Yaghubi H, et al. Prevalence of smoking and its association with health-related behaviours among Iranian university students: a large-scale study. East Mediterr Heal J. 2020;
Bao W, Dar S, Zhu Y, Wu J, Rawal S, Li S, et al. Plasma concentrations of lipids during pregnancy and the risk of gestational diabetes mellitus: a longitudinal study. J Diabetes. 2018;10:487–95.
Ryckman KK, Spracklen CN, Smith CJ, Robinson JG, Saftlas AF. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG An Int J Obstet Gynaecol. 2015;122:643–51.
Jameshorani M, Arefzadeh A, Khalighinejad P, Ranjbar S, Mazloomzadeh S. Evaluation of triglyceride to high-density lipoprotein cholesterol ratio and atherogenic indices in gestational diabetes mellitus. J Pharm Res Int. 2018;22:1–9.
Acknowledgements
The authors wish to thank the Department of Biochemistry and Cell Biology, BUHS and the Department of Genetic Engineering and Biotechnology, University of Rajshahi, Bangladesh, for providing financial support and other facilities during the whole research work. We would like to thank Md. Mominul Islam, Department of Physiology, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh, for his critical review of this article.
Author information
Authors and Affiliations
Contributions
MMO, IAH, MNM; Conceptualization. MMO, IAH, MNM; Methodology. IAH, MNM; Investigation. MMO, SI, MRC; Writing-original draft preparation. MMO, MRC, SI, LA, MNM; Writing-review and editing. MMO, MRC, SI; Manuscript revision. IAH, MNM; Supervision.
Corresponding author
Ethics declarations
Ethics approval
Ethical issue of this study was approved by the ethical review board of the Institute of Biological Sciences (IBSc) of the University of Rajshahi, Rajshahi-6205, Bangladesh, under the certificate number 255(14)/320/IAMEBBC/IBSc. Informed written consent was taken from all patients enrolled in this study.
Consent to participate
The written consent from the participants was taken, describing that the survey report will be published in the journal.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Obaidullah, M.M., Islam, S., Chowdhury, M.R. et al. Correlation analysis of triglycerides to high-density lipoprotein-cholesterol ratio associated with gestational diabetes mellitus. Int J Diabetes Dev Ctries 42, 636–641 (2022). https://doi.org/10.1007/s13410-021-01016-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-021-01016-5



