Abstract
Background
Testicular germ cell tumours (TGCT) are highly sensitive to cisplatin-based chemotherapy, but patients with tumours containing differentiated teratoma components are less responsive to this treatment. The cisplatin sensitivity in TGCT has previously been linked to the embryonic phenotype in the majority of tumours, although the underlying mechanism largely remains to be elucidated. The aim of this study was to investigate the role of the DNA mismatch repair (MMR) system in the cisplatin sensitivity of TGCT.
Methods
The expression pattern of key MMR proteins, including MSH2, MSH6, MLH1 and PMS2, were investigated during testis development and in the pathogenesis of TGCT, including germ cell neoplasia in situ (GCNIS). The TGCT-derived cell line NTera2 was differentiated using retinoic acid (10 μM, 6 days) after which MMR protein expression and activity, as well as cisplatin sensitivity, were investigated in both undifferentiated and differentiated cells. Finally, the expression of MSH2 was knocked down by siRNA in NTera2 cells after which the effect on cisplatin sensitivity was examined.
Results
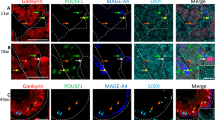
MMR proteins were expressed in proliferating cells in the testes, while in malignant germ cells MMR protein expression was found to coincide with the expression of the pluripotency factor OCT4, with no or low expression in the more differentiated yolk sac tumours, choriocarcinomas and teratomas. In differentiated NTera2 cells we found a significantly (p < 0.05) lower expression of the MMR and pluripotency factors, as well as a reduced MMR activity and cisplatin sensitivity, compared to undifferentiated NTera2 cells. Also, we found that partial knockdown of MSH2 expression in undifferentiated NTera2 cells resulted in a significantly (p < 0.001) reduced cisplatin sensitivity.
Conclusion
This study reports, for the first time, expression of the MMR system in fetal gonocytes, from which GCNIS cells are derived. Our findings in primary TGCT specimens and TGCT-derived cells suggest that a reduced sensitivity to cisplatin in differentiated TGCT components could result from a reduced expression of MMR proteins, in particular MSH2 and MLH1, which are involved in the recognition of cisplatin adducts and in activation of the DNA damage response pathway to initiate apoptosis.






Similar content being viewed by others
References
G. Engholm, J. Ferlay, N. Christensen, F. Bray, M.L. Gjerstorff, A. Klint, J.E. Køtlum, E. Olafsdóttir, E. Pukkala, H.H. Storm, NORDCAN - a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 49, 725–736 (2010)
B. Trabert, J. Chen, S.S. Devesa, F. Bray, K.A. McGlynn, International patterns and trends in testicular cancer incidence, overall and by histologic subtype, 1973-2007. Andrology 3, 4–12 (2015)
T.M. Ulbright, M.B. Amin, B. Balzer, D.M. Berney, J.I. Epstein, C. Guo, M.T. Idrees, L.H.J. Looijenga, G. Paner, E. Rajpert-De Meyts, N.E. Skakkebaek, S.K. Tickoo, A. Yilmaz, J.W. Oosterhuis, in WHO classification of Tumours of the urinary system and male genital organs, 4th edn., ed. by H. Moch, P. A. Humphrey, V. E. Reuter, T. M. Ulbright. Germ cell tumours (IARC Press, Lyon, 2016), pp. 189–226
N.E. Skakkebaek, J.G. Berthelsen, A. Giwercman, J. Müller, Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int. J. Androl. 10, 19–28 (1987)
E. Rajpert-De Meyts, R. Hanstein, N. Jørgensen, N. Graem, P.H. Vogt, N.E. Skakkebaek, Developmental expression of POU5F1 (OCT-3/4) in normal and dysgenetic human gonads. Hum. Reprod. 19, 1338–1344 (2004)
C.E. Hoei-Hansen, J.E. Nielsen, K. Almstrup, S.B. Sonne, N. Graem, N.E. Skakkebaek, H. Leffers, M.E. Rajpert-De, Transcription factor AP-2gamma is a developmentally regulated marker of testicular carcinoma in situ and germ cell tumors. Clin. Cancer Res. 10, 8521–8530 (2004)
C.E. Hoei-Hansen, K. Almstrup, J.E. Nielsen, S. Brask Sonne, N. Graem, N.E. Skakkebaek, H. Leffers, M.E. Rajpert-De, Stem cell pluripotency factor NANOG is expressed in human fetal gonocytes, testicular carcinoma in situ and germ cell tumours. Histopathology 47, 48–56 (2005)
J. de Jong, H. Stoop, G.R. Dohle, C.H. Bangma, M. Kliffen, J.W. van Esser, M. van den Bent, J.M. Kros, J.W. Oosterhuis, L.H. Looijenga, Diagnostic value of OCT3/4 for pre-invasive and invasive testicular germ cell tumours. J. Pathol. 206, 242–249 (2005)
K. Almstrup, C.E. Hoei-Hansen, U. Wirkner, J. Blake, C. Schwager, W. Ansorge, J.E. Nielsen, N.E. Skakkebaek, E. Rajpert-De Meyts, H. Leffers, Embryonic stem cell-like features of testicular carcinoma in situ revealed by genome-wide gene expression profiling. Cancer Res. 64, 4736–4743 (2004)
S.B. Sonne, K. Almstrup, M. Dalgaard, A.S. Juncker, D. Edsgard, L. Ruban, N.J. Harrison, C. Schwager, A. Abdollahi, P.E. Huber, S. Brunak, L.M. Gjerdrum, H.D. Moore, P.W. Andrews, N.E. Skakkebaek, E. Rajpert-De Meyts, H. Leffers, Analysis of gene expression profiles of microdissected cell populations indicates that testicular carcinoma in situ is an arrested gonocyte. Cancer Res. 69, 5241–5250 (2009)
K. Almstrup, J.E. Nielsen, O. Mlynarska, M.T. Jansen, A. Jørgensen, N.E. Skakkebæk, M.E. Rajpert-De, Carcinoma in situ testis displays permissive chromatin modifications similar to immature foetal germ cells. Br. J. Cancer 103, 1269–1276 (2010)
E. Rajpert-De Meyts, K.A. McGlynn, K. Okamoto, M.A. Jewett, C. Bokemeyer, Testicular germ cell tumours. Lancet 387, 1762–1774 (2016)
J.C. Young, S. Wakitani, K.L. Loveland, TGF-β superfamily signaling in testis formation and early male germline development. Semin. Cell Dev. Biol. 45, 94–103 (2015)
S.K. Cheng, F. Olale, A.H. Brivanlou, A.F. Schier, Lefty blocks a subset of TGFbeta signals by antagonizing EGF-CFC coreceptors. PLoS Biol. 2, E30 (2004)
C.M. Spiller, C.W. Feng, A. Jackson, A.J. Gillis, A.D. Rolland, L.H. Looijenga, P. Koopman, J. Bowles, Endogenous Nodal signaling regulates germ cell potency during mammalian testis development. Development 139, 4123–4132 (2012)
D. Nettersheim, S. Jostes, R. Sharma, S. Schneider, A. Hofmann, H.J. Ferreira, P. Hoffmann, G. Kristiansen, M.B. Esteller, H. Schorle, BMP inhibition in seminomas initiates Acquisition of Pluripotency via NODAL signaling resulting in reprogramming to an embryonal carcinoma. PLoS Genet. 11, e1005415 (2015)
R.H. Verhoeven, A. Gondos, M.L. Janssen-Heijnen, K.U. Saum, D.H. Brewster, B. Holleczek, E. Crocetti, S. Rosso, T. Hakulinen, T. Aareleid, H. Brenner, EUNICE Survival Working Group, Testicular cancer in Europe and the USA: survival still rising among older patients. Ann. Oncol 24, 508–513 (2013)
D.J. Higby, H.J. Wallace Jr., D.J. Albert, J.F. Holland, Diaminodichloroplatinum: a phase I study showing responses in testicular and other tumors. Cancer 33, 1219–1225 (1974)
L.H. Einhorn, Curing metastatic testicular cancer. Proc. Natl. Acad. Sci. U. S. A. 99, 4592–4595 (2002)
A. Ioannis, I.A. Voutsadakis, The chemosensitivity of testicular germ cell tumors. Cell. Oncol. 37, 79–94 (2014)
C. Jacobsen, F. Honecker, Cisplatin resistance in germ cell tumours: models and mechanisms. Andrology 3, 111–1121 (2015)
T. Mueller, L.P. Mueller, J. Luetzkendorf, W. Voigt, H. Simon, H.J. Schmoll, Loss of Oct-3/4 expression in embryonal carcinoma cells is associated with induction of cisplatin resistance. Tumour Biol. 27, 71–83 (2006)
R. Koster, A. di Pietro, H. Timmer-Bosscha, J.H. Gibcus, A. van den Berg, A.J. Suurmeijer, R. Bischoff, J.A. Gietema, S. de Jong, Cytoplasmic p21 expression levels determine cisplatin resistance in human testicular cancer. J. Clin. Invest. 120, 3594–3605 (2010)
M. Gutekunst, T. Mueller, A. Weilbacher, M.A. Dengler, J. Bedke, S. Kruck, M. Oren, W.E. Aulitzky, H. van der Kuip, Cisplatin hypersensitivity of testicular germ cell tumors is determined by high constitutive Noxa levels mediated by Oct-4. Cancer Res. 73, 1460–1469 (2013)
L. Grande, G. Bretones, M. Rosa-Garrido, E.M. Garrido-Martin, T. Hernandez, S. Fraile, L. Botella, E. de Alava, A. Vidal, X. Garcia del Muro, A. Villanueva, M.D. Delgado, J.L. Fernandez-Luna, Transcription factors Sp1 and p73 control the expression of the proapoptotic protein NOXA in the response of testicular embryonal carcinoma cells to cisplatin. J. Biol. Chem. 287, 26495–26505 (2012)
J.S. Kerley-Hamilton, A.M. Pike, N. Li, J. DiRenzo, M.J. Spinella, A p53-dominant transcriptional response to cisplatin in testicular germ cell tumor-derived human embryonal carcinoma. Oncogene 24, 6090–6100 (2005)
J. Bartkova, Z. Horejsí, M. Sehested, J.M. Nesland, E. Rajpert-De Meyts, N.E. Skakkebaek, M. Stucki, S. Jackson, J. Lukas, J. Bartek, DNA damage response mediators MDC1 and 53BP1: constitutive activation and aberrant loss in breast and lung cancer, but not in testicular germ cell tumours. Oncogene 26, 7414–7422 (2007)
M. Gutekunst, M. Oren, A. Weilbacher, M.A. Dengler, C. Markwardt, J. Thomale, W.E. Aulitzky, H. van der Kuip, p53 hypersensitivity is the predominant mechanism of the unique responsiveness of testicular germ cell tumor (TGCT) cells to cisplatin. PLoS One 6, e19198 (2011)
J.R. Masters, B. Köberle, Curing metastatic cancer: lessons from testicular germ-cell tumours. Nat. Rev. Cancer 3, 517–525 (2003)
F. Honecker, H. Wermann, F. Mayer, A.J. Gillis, H. Stoop, R.J. van Gurp, K. Oechsle, E. Steyerberg, J.T. Hartmann, W.N. Dinjens, J.W. Oosterhuis, C. Bokemeyer, L.H. Looijenga, Microsatellite instability, mismatch repair deficiency, and BRAF mutation in treatment-resistant germ cell tumors. J. Clin. Oncol. 27, 2129–2136 (2009)
R.E. Velasco, M. Schultz, I.I. Wistuba, L. Villarroel, M.S. Koh, F.S. Leach, Microsatellite instability and loss of heterozygosity have distinct prognostic value for testicular germ cell tumor recurrence. Cancer Biol. Ther 3, 1152–1158 (2004a)
A. Velasco, E. Riquelme, M. Schultz, I.I. Wistuba, L. Villarroel, J. Pizarro, A. Berlin, M. Ittmann, M.S. Koh, F.S. Leach, Mismatch repair gene expression and genetic instability in testicular germ cell tumor. Cancer Biol Ther 3, 977–982 (2004b)
A. Velasco, A. Corvalan, I.I. Wistuba, E. Riquelme, R. Chuaqui, A. Majerson, F.S. Leach, Mismatch repair expression in testicular cancer predicts recurrence and survival. Int. J. Cancer 122, 1774–1777 (2008)
M. Kansikas, M. Kasela, J. Kantelinen, M. Nyström, Assessing how reduced expression levels of the mismatch repair genes MLH1, MSH2, and MSH6 affect repair efficiency. Hum. Mutat. 35, 1123–1127 (2014)
S. Haraldsdottir, H. Hampel, J. Tomsic, W.L. Frankel, R. Pearlman, A. de la Chapelle, C.C. Pritchard, Colon and endometrial cancers with mismatch repair deficiency can arise from somatic, rather than germline, mutations. Gastroenterology 147, 1308–1316 (2014)
J.A. Mello, S. Acharya, R. Fishel, J.M. Essigmann, The mismatch-repair protein hMSH2 binds selectively to DNA adducts of the anticancer drug cisplatin. Chem. Biol. 3, 579–589 (1996)
R.P. Topping, J.C. Wilkinson, K.D. Scarpinato, Mismatch repair protein deficiency compromises cisplatin-induced apoptotic signaling. J. Biol. Chem. 284, 14029–14039 (2009)
A. Sawant, A. Kothandapani, A. Zhitkovich, R.W. Sobol, S.M. Patrick, Role of mismatch repair proteins in the processing of cisplatin interstrand cross-links. DNA Repair 5, 126–136 (2015)
M. Li, Mechanisms and functions of DNA mismatch repair. Cell Res. 18, 85–98 (2008)
N. Pabla, Z. Ma, M.A. McIlhatton, R. Fishel, Z. Dong, hMSH2 recruits ATR to DNA damage sites for activation during DNA damage-induced apoptosis. J. Biol. Chem. 286, 10411–10418 (2011)
J. Jia, Z. Wang, J. Cai, Y. Zhang, PMS2 expression in epithelial ovarian cancer is posttranslationally regulated by Akt and essential for platinum-induced apoptosis. Tumour Biol. 37, 3059–3069 (2016)
M.R. Maduro, R. Casella, E. Kim, N. Lévy, C. Niederberger, L.I. Lipshultz, D.J. Lamb, Microsatellite instability and defects in mismatch repair proteins: a new aetiology for Sertoli cell-only syndrome. Mol. Hum. Reprod. 9, 61–68 (2003)
F. Mayer, A.J. Gillis, W. Dinjens, J.W. Oosterhuis, C. Bokemeyer, L.H. Looijenga, Microsatellite instability of germ cell tumors is associated with resistance to systemic treatment. Cancer Res. 62, 2758–2760 (2002)
A. Jørgensen, J.E. Nielsen, M. Blomberg Jensen, N. Græm, M.E. Rajpert-De, Analysis of meiosis regulators in human gonads: a sexually dimorphic spatio-temporal expression pattern suggests involvement of DMRT1 in meiotic entry. Mol. Hum. Reprod. 18, 523–534 (2012)
A. Jørgensen, M. Blomberg Jensen, J.E. Nielsen, A. Juul, M.E. Rajpert-De, Influence of vitamin D on cisplatin sensitivity in testicular germ cell cancer-derived cell lines and in a NTera2 xenograft model. J. Steroid Biochem. Mol. Biol. 136, 238–246 (2013a)
A. Jørgensen, J.E. Nielsen, K. Almstrup, B.G. Toft, B.L. Petersen, M.E. Rajpert-De, Dysregulation of the mitosis-meiosis switch in testicular carcinoma in situ. J. Pathol. 229, 588–598 (2013b)
T. Svingen, A. Jørgensen, M.E. Rajpert-De, Validation of endogenous normalizing genes for expression analyses in adult human testis and germ cell neoplasms. Mol. Hum. Reprod. 20, 709–718 (2014)
A. Jørgensen, J. Young, J.E. Nielsen, U.N. Joensen, B.G. Toft, E. Rajpert-De Meyts, K.L. Loveland, Hanging drop cultures of human testis and testis cancer samples: a model used to investigate activin treatment effects in a preserved niche. Br. J. Cancer 110, 2604–2614 (2014)
D.C. Thomas, J.D. Roberts, T.A. Kunkel, Heteroduplex repair in extracts of human HeLa cells. J. Biol. Chem. 266, 3744–3751 (1991)
P.W. Andrews, Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev. Biol. 103, 285–293 (1984)
J. Olasz, L. Mándoky, L. Géczi, I. Bodrogi, O. Csuka, M. Bak, Influence of hMLH1 methylation, mismatch repair deficiency and microsatellite instability on chemoresistance of testicular germ-cell tumors. Anticancer Res. 25, 4319–4324 (2005)
M. Devouassoux-Shisheboran, C. Mauduit, R. Bouvier, F. Berger, M. Bouras, J.P. Droz, M. Benahmed, Expression of hMLH1 and hMSH2 and assessment of microsatellite instability in testicular and mediastinal germ cell tumours. Mol. Hum. Reprod. 7, 1099–1105 (2001)
C.J. Giuliano, S.J. Freemantle, M.J. Spinella, Testicular germ cell tumors: a paradigm for the successful treatment of solid tumor stem cells. Curr Cancer Ther Rev 2, 255–270 (2006)
F.R. Salsbury Jr., J.E. Clodfelter, M.B. Gentry, T. Hollis, K.D. Scarpinato, The molecular mechanism of DNA damage recognition by MutS homologs and its consequences for cell death response. Nucleic Acids Res. 34, 2173–2185 (2006)
G. Marra, S. D'Atri, H. Yan, C. Perrera, E. Cannavo’, B. Vogelstein, J. Jiricny, Phenotypic analysis of hMSH2 mutations in mouse cells carrying human chromosomes. Cancer Res. 61, 7719–7721 (2001)
F. Mayer, H. Stoop, G.L. Scheffer, R. Scheper, J.W. Oosterhuis, L.H. Looijenga, C. Bokemeyer, Molecular determinants of treatment response in human germ cell tumors. Clin. Cancer Res. 9, 767–773 (2003)
S.M. Baker, C.E. Bronner, L. Zhang, A.W. Plug, M. Robatzek, G. Warren, E.A. Elliott, J. Yu, T. Ashley, N. Arnheim, R.A. Flavell, R.M. Liskay, Male mice defective in the DNA mismatch repair gene PMS2 exhibit abnormal chromosome synapsis in meiosis. Cell 82, 309–319 (1995)
S.M. Baker, A.W. Plug, T.A. Prolla, C.E. Bronner, A.C. Harris, X. Yao, D.M. Christie, C. Monell, N. Arnheim, A. Bradley, T. Ashley, R.M. Liskay, Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat. Genet. 13, 336–342 (1996)
A. Svetlanov, P.E. Cohen, Mismatch repair proteins, meiosis, and mice: understanding the complexities of mammalian meiosis. Exp. Cell Res. 296, 71–79 (2004)
W.M. Baarends, R. van der Laan, J.A. Grootegoed, DNA repair mechanisms and gametogenesis. Reproduction 121, 31–39 (2001)
Acknowledgements
This work was supported by the Danish Cancer Society (R72-A4335-13-S2) to AJ. Additional funding for this project was obtained from the Nordea-fonden to LJR, the China Scholarship Council (CSC) to DL and the Research Fund at Rigshospitalet to JEN. The authors wish to thank the staff members at the Department of Pathology and Urology for help with collection of tissue. The excellent technical assistance of Camilla Tang Thomsen, Ana Ricci Nielsen, Brian Vendelbo Hansen and Betina F. Nielsen is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Rudolph, C., Melau, C., Nielsen, J.E. et al. Involvement of the DNA mismatch repair system in cisplatin sensitivity of testicular germ cell tumours. Cell Oncol. 40, 341–355 (2017). https://doi.org/10.1007/s13402-017-0326-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-017-0326-8