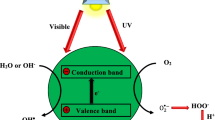
Abstract
The paper and pulp industry stands out to be one of the industries that are growing at a fast pace. Besides their characteristics such as high water consumption of 695.7 million m3 per year and subsequent high energy consumption, there are plenty of environmental impacts arising from this industry. Approximately 300 different types of harmful organic pollutants such as phenols, dioxins, and other organic substances are the significant effluents of the paper and pulp industry. As per the regulations set by the world health organization (WHO), the permissible limit of such pollutants is less than 1 ppm. Since the complete degradation of these pollutants is difficult to achieve using conventional techniques, advanced oxidation processes (AOPs) are widely being used as promising alternatives. On the other hand, depleting non-renewable energy resources are forcing industries to look for energy-efficient technologies. Photocatalysis is one such AOPs, which has the potential to solve the energy-wastewater nexus and is the primary focus of this review in terms of waste treatment from the paper and pulp industry. The photocatalytic materials induce photoreactions by receiving photon energy from light. However, harnessing light energy to the fullest is a challenge owing to the limited share of UV spectrum in sunlight. Different composite photocatalysts at different loadings, pollutant concentrations, and contact time along with their respective efficiencies are reported. The enhancement in thermodynamic driving force due to shift in Fermi energy levels with a suitable example is explained. The dependence of photocatalysis on various factors such as organic species, temperature, and light intensity makes it tough to predict the precise kinetic model in real-life experiments. The hydrogen production from the degradation of organic pollutants was discussed vividly and by optimization of the processes, an estimated amount of 0.45% of the total world’s energy could be produced from the paper and pulp industry alone.














Similar content being viewed by others
Data availability
Not applicable.
References
Martin Hagberg Börjesson EOA (2015) No Title. IEA
Martin J, Haggith M (2018) The state of the global paper industry- shifting seas: New challenges and opportunities for forests, people and the climate- environmental paper network
Kulkarni HD (2013) pulp and paper industry raw material scenario-ITC plantation a case study. IPPTA 25:79–89
CPCB (2005) Status of Sewage Treatment in India. Cent Pollut Control Board:1–97
Pandey A, Panwar S, Siddiqui NA, Endlay N (2012) Adsorbable Organichalide (AOX) level and their reduction by chemical treatment of bleach plant effluent of agro and wood-based pulp and paper mills. J Ind Pollut Control 28:165–170
Gergov M, Priha M, Talka E, Valttila O, Kangas A, Kukkonen K (1988) chlorinated organic compounds in effluent treatment at kraft mills. TAPPI 71:175–184
RSPCA Slaughter Fact File. Rspca
Bajpai P (2013) bleach plant effluents from pulp and paper industry
ESCWA - United Nations (2003) Waste-Water Treatment Technologies : a General Review. Econ Soc Comm West Asia 1320
U.S. EPA (2000) Wastewater Technology Fact Sheet Package Plants. United States Environ Prot Agency 1–7. EPA 832-F-99-062
Subashini LM (2015) Review on Biological Treatment processes of Pulp and Paper Industry Waste Water. 3721–3725. 10.15680/IJIRSET.2015.0405195
Pokhrel D, Viraraghavan T (2004) Treatment of pulp and paper mill wastewater - a review. Sci. Total Environ. 333:37–58
Kimura K, Hane Y, Watanabe Y et al (2004) Irreversible membrane fouling during ultrafiltration of surface water. Water Res 38:3431–3441. https://doi.org/10.1016/j.watres.2004.05.007
Program FC, Preethi V, Kanmani S et al (2015) Electrochemical technologies in wastewater treatment. Top Catal 12:1–7. https://doi.org/10.1063/1.2977969
Oturan MA, Aaron JJ (2014) Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit Rev Environ Sci Technol 44:2577–2641. https://doi.org/10.1080/10643389.2013.829765
Calace N, Nardi E, Petronio BM, Pietroletti M (2002) Adsorption of phenols by papermill sludges. Environ Pollut 118:315–319. https://doi.org/10.1016/S0269-7491(01)00303-7
Li X, Yu J, Wageh S et al (2016) Graphene in photocatalysis: a review. Small 12:6640–6696. https://doi.org/10.1002/smll.201600382
Pirkanniemi K, Sillanpää M (2002) Heterogeneous water phase catalysis as an environmental application: a review. Chemosphere 48:1047–1060. https://doi.org/10.1016/S0045-6535(02)00168-6
Turchi CS, Ollis DF (1990) Photocatalytic degradation of organic water contaminants: mechanisms involving hydroxyl radical attack. J Calatysis 122:178–192. https://doi.org/10.1016/0021-9517(90)90269-P
Hoffmann MR, Martin ST, Choi W, Bahnemann DW (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95:69–96. https://doi.org/10.1021/cr00033a004
Braham RJ, Harris AT (2009) Review of major design and scale-up considerations for solar photocatalytic reactors. Ind Eng Chem Res 48:8890–8905. https://doi.org/10.1021/ie900859z
Khalil LB, Mourad WE, Rophael MW (1998) Photocatalytic reduction of environmental pollutant Cr(VI) over some semiconductors under UV/visible light illumination. Appl Catal B Environ 17:267–273. https://doi.org/10.1016/S0926-3373(98)00020-4
Khalil LB, Rophael MW, Mourad WE (2002) The removal of the toxic Hg (II) salts from water by photocatalysis. Appl Catal B Environ 36:125–130
Dahl M, Liu Y, Yin Y (2014) Composite Titanium Dioxide Nanomaterials.pdf. Chem Rev 114:985–9889. https://doi.org/10.1021/cr400634p
Hernández-Alonso MD, Fresno F, Suárez S, Coronado JM (2009) Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ Sci 2:1231. https://doi.org/10.1039/b907933e
Hong E, Kim D, Kim JH (2014) Heterostructured metal sulfide (ZnS-CuS-CdS) photocatalyst for high electron utilization in hydrogen production from solar water splitting. J Ind Eng Chem 20:3869–3874. https://doi.org/10.1016/j.jiec.2013.12.092
Yue M, Wang R, Cheng N et al (2016) ZnCr2S4: Highly effective photocatalyst converting nitrate into N2 without over-reduction under both UV and pure visible light. Sci Rep 6:1–11. https://doi.org/10.1038/srep30992
Chen D, Huang F, Ren G et al (2010) ZnS nano-architectures: photocatalysis, deactivation and regeneration. Nanoscale 2:2062. https://doi.org/10.1039/c0nr00171f
Han C, Yang M-Q, Weng B, Xu Y-J (2014) Improving the photocatalytic activity and anti-photocorrosion of semiconductor ZnO by coupling with versatile carbon. Phys Chem Chem Phys 16:16891. https://doi.org/10.1039/C4CP02189D
Balandin AA, Ghosh S, Bao W et al (2008) Superior thermal conductivity of single-layer graphene. Nano Lett 8:902–907. https://doi.org/10.1021/nl0731872
Balandin AA (2011) Thermal properties of graphene and nanostructured carbon materials. Nat Mater 10:569–581. https://doi.org/10.1038/nmat3064
Du X, Skachko I, Barker A, Andrei EY (2008) Approaching ballistic transport in suspended graphene. Nat Nanotechnol 3:491–495. https://doi.org/10.1038/nnano.2008.199
Bolotin KI, Sikes KJ, Jiang Z et al (2008) Ultrahigh electron mobility in suspended graphene. Solid State Commun 146:351–355. https://doi.org/10.1016/j.ssc.2008.02.024
Yang J, Zhang J, Zhu L et al (2006) Synthesis of nano titania particles embedded in mesoporous SBA-15: Characterization and photocatalytic activity. J Hazard Mater 137:952–958. https://doi.org/10.1016/j.jhazmat.2006.03.017
Aronson BJ, Blanford CF, Stein A (1997) Solution-Phase Grafting of Titanium Dioxide onto the Pore Surface of Mesoporous Silicates: Synthesis and Structural Characterization. Chem Mater 9:2842–2851. https://doi.org/10.1021/cm970180k
Wang Z, Zhang F, Yang Y et al (2007) Facile Postsynthesis of Visible-Light-Sensitive Titanium Dioxide / Mesoporous SBA-15 Facile Postsynthesis of Visible-Light-Sensitive Titanium Dioxide / Mesoporous SBA-15. Chem Mater 19:3286–3293. https://doi.org/10.1021/cm062041l
Hernandez RA (2015) Photocatalytic semi conductors, 1st edn. Springer
Kumar A, Yadav N, Bhatt M et al (2015) Sol-Gel derived nanomaterials and it’s applications: a review. Res J Chem Sci 5:98–105
Neiderberger M, Pinna N (2009) Metal oxide nano particles in organic solvents. Springer-Verlag, London
Nirmala Jothi NS, Dennis Christy P, Baby Suganthi AR et al (2011) Development of CdS nanorods of high aspect ratio under hydrothermal conditions with PEG template. J Cryst Growth 316:126–131. https://doi.org/10.1016/j.jcrysgro.2010.12.055
Ilango MS, Mutalikdesai A, Ramasesha SK (2016) Anodization of Aluminium using a fast two-step process. J Chem Sci 128:153–158. https://doi.org/10.1007/s12039-015-1006-8
Pardeshi SK, Patil AB (2008) A simple route for photocatalytic degradation of phenol in aqueous zinc oxide suspension using solar energy. Sol Energy 82:700–705. https://doi.org/10.1016/j.solener.2008.02.007
Sonawane RS, Dongare MK (2006) Sol-gel synthesis of Au/TiO2thin films for photocatalytic degradation of phenol in sunlight. J Mol Catal A Chem 243:68–76. https://doi.org/10.1016/j.molcata.2005.07.043
Dewidar H, Nosier SA, El-Shazly AH (2017) Photocatalytic degradation of phenol solution using Zinc Oxide/UV. J Chem Heal Saf:1–10. https://doi.org/10.1016/j.jchas.2017.06.001
Malekshoar G, Pal K, He Q et al (2014) Enhanced Solar Photocatalytic Degradation of Phenol with Coupled Graphene-Based Titanium Dioxide and Zinc Oxide. Ind Eng Chem Res 53:18824–18832. https://doi.org/10.1021/ie501673v
Yuan Z, Jia J, Zhang L (2002) Influence of co-doping of Zn(II)+Fe(III) on the photocatalytic activity of TiO2 for phenol degradation. Mater Chem Phys 73:323–326. https://doi.org/10.1016/S0254-0584(01)00373-X
Naeem K, Ouyang F (2010) Preparation of Fe3+-doped TiO2 nanoparticles and its photocatalytic activity under UV light. Phys B Condens Matter 405:221–226. https://doi.org/10.1016/j.physb.2009.08.060
Chiou CH, Juang RS (2007) Photocatalytic degradation of phenol in aqueous solutions by Pr-doped TiO2 nanoparticles. J Hazard Mater 149:1–7. https://doi.org/10.1016/j.jhazmat.2007.03.035
Liqiang J, Xiaojun S, Baifu X et al (2004) The preparation and characterization of la doped TiO2 nanoparticles and their photocatalytic activity. J Solid State Chem 177:3375–3382. https://doi.org/10.1016/j.jssc.2004.05.064
Chowdhury P, Moreira J, Gomaa H, Ray AK (2012) Visible-solar-light-driven photocatalytic degradation of phenol with dye-sensitized TiO2: parametric and kinetic study. Ind Eng Chem Res 51:4523–4532. https://doi.org/10.1021/Ie2025213
Yunus NN, Hamzah F, So’aib MS, Krishnan J (2017) Effect of Catalyst Loading on Photocatalytic Degradation of Phenol by Using N, S Co-doped TiO 2. IOP Conf Ser Mater Sci Eng 206:012092. https://doi.org/10.1088/1757-899X/206/1/012092
Benhebal H, Chaib M, Salmon T et al (2013) Photocatalytic degradation of phenol and benzoic acid using zinc oxide powders prepared by the sol-gel process. Alexandria Eng J 52:517–523. https://doi.org/10.1016/j.aej.2013.04.005
Shaban YA, El Sayed MA, El Maradny AA et al (2013) Photocatalytic degradation of phenol in natural seawater using visible light active carbon modified (CM)-n-TiO2nanoparticles under UV light and natural sunlight illuminations. Chemosphere 91:307–313. https://doi.org/10.1016/j.chemosphere.2012.11.035
Rafiee E, Noori E, Zinatizadeh AA, Zanganeh H (2016) Photocatalytic degradation of phenol using a new developed TiO 2 /graphene/heteropoly acid nanocomposite: synthesis, characterization and process optimization. RSC Adv 6:96554–96562. https://doi.org/10.1039/C6RA09897E
Al-Hamdi AM, Sillanpää M, Dutta J (2015) Photocatalytic degradation of phenol by iodine doped tin oxide nanoparticles under UV and sunlight irradiation. J Alloys Compd 618:366–371. https://doi.org/10.1016/j.jallcom.2014.08.120
Yin W, Wang W, Sun S (2010) Photocatalytic degradation of phenol over cage-like Bi2MoO6hollow spheres under visible-light irradiation. Catal Commun 11:647–650. https://doi.org/10.1016/j.catcom.2010.01.014
Long M, Cai W, Cai J et al (2006) Efficient Photocatalytic Degradation of Phenol over Co 3 O 4 /BiVO 4 Composite under Visible Light Irradiation. J Phys Chem B 110:20211–20216. https://doi.org/10.1021/jp063441z
Wang P-Q, Bai Y, Liu J-Y (2013) Photocatalytic degradation of phenol using Au/Bi2WO6 composite microspheres under visible-light irradiation. Micro Nano Lett 8:90–93. https://doi.org/10.1049/mnl.2012.0759
Yang J, Hu R, Meng W, Du Y (2016) A novel p-LaFeO 3 /n-Ag 3 PO 4 heterojunction photocatalyst for phenol degradation under visible light irradiation. Chem Commun 52:2620–2623. https://doi.org/10.1039/C5CC09222A
Wang H, Liu Y, Li M et al (2010) Multifunctional TiO2nanowires-modified nanoparticles bilayer film for 3D dye-sensitized solar cells. Optoelectron Adv Mater Rapid Commun 4:1166–1169. https://doi.org/10.1039/b000000x
Xu J, Wang F, Liu W, Cao W (2013) Nanocrystalline N-Doped TiO2 Powders: Mild Hydrothermal Synthesis and Photocatalytic Degradation of Phenol under Visible Light Irradiation. Int J Photoenergy 2013:1–7. https://doi.org/10.1155/2013/616139
Borji SH, Nasseri S, Mahvi AH et al (2014) Investigation of photocatalytic degradation of phenol by Fe ( III ) -doped TiO 2 and TiO 2 nanoparticles. J Environ Heal Sci Eng:1–10. https://doi.org/10.1186/2052-336X-12-101
Tian F, Zhu R, Song K et al (2016) Synergistic photocatalytic degradation of phenol using precious metal supported titanium dioxide with hydrogen peroxide. Environ Eng Sci 33:185–192. https://doi.org/10.1089/ees.2014.0153
Khraisheh M, Wu L, Al-Muhtaseb AH et al (2012) Phenol degradation by powdered metal ion modified titanium dioxide photocatalysts. Chem Eng J 213:125–134. https://doi.org/10.1016/j.cej.2012.09.108
Boonprakob N, Chomkitichai W, Ketwaraporn J et al (2017) Photocatalytic degradation of phenol over highly visible-light active BiOI/TiO<inf>2</inf> nanocomposite photocatalyst. Eng J 21. https://doi.org/10.4186/ej.2017.21.1.81
Shukla PR, Wang S, Ang HM, Tadé MO (2010) Photocatalytic oxidation of phenolic compounds using zinc oxide and sulphate radicals under artificial solar light. Sep Purif Technol 70:338–344. https://doi.org/10.1016/j.seppur.2009.10.018
Zaidan LEMC, Rodriguez-Díaz JM, Napoleão DC et al (2017) Heterogeneous photocatalytic degradation of phenol and derivatives by (BiPO4/H2O2/UV and TiO2/H2O2/UV) and the evaluation of plant seed toxicity tests. Korean J Chem Eng 34:511–522. https://doi.org/10.1007/s11814-016-0293-1
Lathasree S, Rao AN, Sivasankar B et al (2004) Heterogeneous photocatalytic mineralisation of phenols in aqueous solutions. J Mol Catal A Chem 223:101–105. https://doi.org/10.1016/j.molcata.2003.08.032
Morales-Flores N, Pal U, Sánchez Mora E (2011) Photocatalytic behavior of ZnO and Pt-incorporated ZnO nanoparticles in phenol degradation. Appl Catal A Gen 394:269–275. https://doi.org/10.1016/j.apcata.2011.01.011
Feng X, Lou X (2015) The effect of surfactants-bound magnetite (Fe<inf>3</inf>O<inf>4</inf>) on the photocatalytic properties of the heterogeneous magnetic zinc oxides nanoparticles. Sep Purif Technol 147:266–275. https://doi.org/10.1016/j.seppur.2015.04.036
So’aib MS, Mazlan N (2015) Degradation of Phenol from Glove Factory’s Effluent by Zn/TiO2 Photocatalyst. Adv Mater Res 1113:528–533. https://doi.org/10.4028/www.scientific.net/AMR.1113.528
Mureseanu M, Parvulescu V, Radu T et al (2015) Mesoporous CeTiSiMCM-48 as novel photocatalyst for degradation of organic compounds. J Alloys Compd 648:864–873. https://doi.org/10.1016/j.jallcom.2015.07.078
Sherly ED, Judith Vijaya J, John Kennedy L (2015) Visible-light-induced photocatalytic performances of ZnO-CuO nanocomposites for degradation of 2,4-dichlorophenol. Cuihua Xuebao/Chinese J Catal 36:1263–1272. https://doi.org/10.1016/S1872-2067(15)60886-5
Khezrianjoo S, Revanasiddappa H (2012) Langmuir-Hinshelwood Kinetic Expression for the Photocatalytic Degradation of Metanil Yellow Aqueous Solutions by ZnO Catalyst. Chem Sci J 2012:85–85
Umar M, Abdul H (2013) Photocatalytic degradation of organic pollutants in water. Org Pollut Monit Risk Treat. https://doi.org/10.5772/53699
Liu B, Zhao X, Terashima C et al (2014) Thermodynamic and kinetic analysis of heterogeneous photocatalysis for semiconductor systems. Phys Chem Chem Phys 16:8751. https://doi.org/10.1039/c3cp55317e
Christoforidis KC, Fornasiero P (2017) Photocatalytic hydrogen production: a rift into the future energy supply. ChemCatChem 9:1523–1544. https://doi.org/10.1002/cctc.201601659
Yilmaz F, Balta MT, Selbaş R (2016) A review of solar based hydrogen production methods. Renew Sustain Energy Rev 56:171–178. https://doi.org/10.1016/j.rser.2015.11.060
Ren J, Gao S, Tan S et al (2015) Role prioritization of hydrogen production technologies for promoting hydrogen economy in the current state of China. Renew Sustain Energy Rev 41:1217–1229. https://doi.org/10.1016/j.rser.2014.09.028
iea (2015) Technology Roadmap. SpringerReference 81. https://doi.org/10.1007/SpringerReference_7300
Voldsund M, Jordal K, Anantharaman R (2016) Hydrogen production with CO2capture. Int J Hydrogen Energy 41:4969–4992. https://doi.org/10.1016/j.ijhydene.2016.01.009
Preethi V, Kanmani S (2013) Photocatalytic hydrogen production. Mater Sci Semicond Process 16:561–575. https://doi.org/10.1016/j.mssp.2013.02.001
Liu R, Yoshida H, Ichiro FS, Arai M (2014) Photocatalytic hydrogen production from glycerol and water with NiOx/TiO2catalysts. Appl Catal B Environ 144:47–45. https://doi.org/10.1016/j.apcatb.2013.06.024
Zou X, Zhang Y (2015) Noble metal-free hydrogen evolution catalysts for water splitting. Chem Soc Rev 44:5148–5180. https://doi.org/10.1039/C4CS00448E
Zhang J, Qi L, Ran J et al (2014) Ternary NiS/ZnxCd1-xS/reduced graphene oxide nanocomposites for enhanced solar photocatalytic h2-production activity. Adv Energy Mater 4:2–7. https://doi.org/10.1002/aenm.201301925
Stegbauer L, Schwinghammer K, Lotsch BV (2014) A hydrazone-based covalent organic framework for photocatalytic hydrogen production. Chem Sci 5:2789–2793. https://doi.org/10.1039/C4SC00016A
Sprick RS, Bonillo B, Clowes R et al (2016) Visible-light-driven hydrogen evolution using planarized conjugated polymer photocatalysts. Angew Chemie Int Ed 55:1792–1796. https://doi.org/10.1002/anie.201510542
Jiang D, Sun Z, Jia H et al (2016) A cocatalyst-free CdS nanorod/ZnS nanoparticle composite for high-performance visible-light-driven hydrogen production from water. J Mater Chem A 4:675–683. https://doi.org/10.1039/C5TA07420G
Chen Y, Zhao S, Wang X et al (2016) Synergetic integration of Cu1.94S-ZnxCd1-xS heteronanorods for enhanced visible-light-driven photocatalytic hydrogen production. J Am Chem Soc 138:4286–4289. https://doi.org/10.1021/jacs.5b12666
Wen J, Li X, Li H et al (2015) Enhanced visible-light H2evolution of g-C3N4photocatalysts via the synergetic effect of amorphous NiS and cheap metal-free carbon black nanoparticles as co-catalysts. Appl Surf Sci 358:204–212. https://doi.org/10.1016/j.apsusc.2015.08.244
Zhong Y, Yuan J, Wen J et al (2015) Earth-abundant NiS co-catalyst modified metal-free mpg-C 3 N 4 /CNT nanocomposites for highly efficient visible-light photocatalytic H 2 evolution. Dalt Trans 44:18260–18269. https://doi.org/10.1039/C5DT02693H
Zheng D, Huang C, Wang X (2015) Post-annealing reinforced hollow carbon nitride nanospheres for hydrogen photosynthesis. Nanoscale 7:465–470. https://doi.org/10.1039/C4NR06011C
Kim J, Choi W (2010) Hydrogen producing water treatment through solar photocatalysis. Energy Environ Sci 3:1042. https://doi.org/10.1039/c003858j
Program FC (2016) • History • Progress • Future # H2AMR # H2IQ
Demirel Y (2016) Energy Sources. In: Energy: Production, Conversion, Storage, Conservation, and Coupling, pp, 35–71
Acknowledgements
We would like to thank the Director of BITS Pilani, K. K. Birla Goa Campus, for support in using the institutional infrastructure during the preparation of this paper.
Funding
The authors received funding for this work from SERB-DST (EMR/2016/003370), the Government of India.
Author information
Authors and Affiliations
Contributions
Aashish Moses: Conceptualization, methodology, data curation, writing-original draft preparation.
Janaki Komandur: data curation, validation, writing-reviewing and editing.
Dileep Maarisetty: data curation, validation, writing-reviewing and editing.
Priyabrat Mohapatra: supervision, writing-reviewing and editing.
Saroj Sundar Baral: supervision, visualization, investigation, data curation, writing-original draft preparation.
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property. We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs.

Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moses, A., Komandur, J., Maarisetty, D. et al. A review on photocatalytic hydrogen production potential from paper and pulp industry wastewater. Biomass Conv. Bioref. 14, 3135–3159 (2024). https://doi.org/10.1007/s13399-022-02658-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02658-z