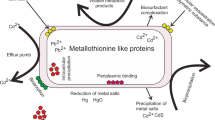
Abstract
The widespread use of hexavalent chromium Cr(VI) in the leather industry causes substantial environmental problems when effluents are left untreated. Therefore, the present work attempts to assess the ability of Bacillus thuringiensis (V45) and Staphylococcus capitis (S21), isolated from tannery industry sediment, to detoxify Cr(VI) by reducing the oxidation state. Initially, the minimum tolerance of chromium by both bacteria was found up to 1000 μg/mL. V45 could tolerate Cr(VI) (520 μg/mL), and S21 could also tolerate hexavalent Cr(VI) (340 μg/mL). Similarly, both bacteria were able to tolerate other metals such as Hg2+ (40 μg/mL), Cu2+ (30 μg/mL), Ni2+ (60 μg/mL), Zn2+ (40 μg/mL), and Pb2+ (30 μg/mL). V45 and S21 could decrease Cr(VI) at a primary concentration of 50 μg/mL up to 86.42% and 97.34%, respectively. In optimization experiments, the best temperature to decrease Cr(VI) was shown to be 35 °C with pH 7 for 96 h. The occurrence of Cu2+ and Na+ slightly increased during the decrease of hexavalent Cr(VI) by V45, while the isolate S21 exhibited the same effects with Cu2+, Mn2+, and Na+. The carboxylate and amino conjugates in the biomass are intricate in the bioreduction of Cr(VI), as confirmed by FTIR spectroscopy. In addition, SEM imagery revealed the accumulation of Cr(VI) around both types of bacterial cells. The occurrence of other elements was evident from SEM-EDS spectroscopy. This study demonstrated the ability of native bacterial populations (V45 and S21) in tannery sediment to reduce Cr(VI) compounds.
Graphical abstract

Similar content being viewed by others
1 Introduction
Chromium contamination in soil and groundwater generally results from the expulsion of wastes containing chromium (VI) via wastewater from tanneries, which is due to its wide use in the leather industry for the stabilization of the leather. Similarly, chromium is used in the manufacture of alloys, wood preservation, metal plating, steel, and pigmentation [1]. Chromium is present in the ecosystem as Cr(VI) and Cr(III) in oxidation states with dissimilar toxicity and mobility features [2]. The Cr(VI) occurs naturally as the chromate oxyanion (CrO42−), which is readily soluble in soil and water, and can percolate in the environment [3]. Moreover, the biological uptake of Cr(VI) leads to acute toxicity; it is also teratogenic and a carcinogen [4]. The toxicity of Cr(VI) causes severe illnesses and disorders in humans due to its carcinogenic properties [5]. Generally, trivalent chromium is less accessible for biological use due to the lower solubility of the hydroxide (Cr(OH)3) and formation of strong adsorptive bonds between soil materials and chromium. Therefore, significant remediation techniques are followed to reduce the redox state of chromium from Cr(VI) to Cr(III) to minimize its toxicity [6].
Literature consists of conservative practices for eliminating leached heavy metals like Cr(IV) through carbon adsorption and reverse osmosis, physicochemical process, solvent extraction, ion exchange, membrane separation, electrocoagulation, dialysis/electrodialysis and adsorption [7,8,9], and ion conversation [10]. These procedures are unsuccessful when dealing with ions of heavy metal with low strength and require positive shortcomings, which comprise the assembly of subordinate waste produced through a sequence of treatments. The fact that Cr(VI) is a sturdy oxidizing agent may affect the stability of ion exchange resin [11] and results in maximum operational and maintenance costs, greater working difficulty, requirement for more energy input, low efficiency, and inadequate metal removal [12]. Plant-based remediation of chromium is an alternate technique that is laborious but more economical than some other protocols. However, phytoremediation is a time-consuming process, and it is necessary to grow the precise plant species. Therefore, it is necessary to search for a newer and more efficient technology to remove toxic chromium from the environment. To this end, treatments of biological or microbial origin as the choice for the detoxification of Cr(VI) have received much attention. These treatments potentially have several advantages such as time saving, high efficiency, lower processing cost, and not producing a residual toxic sludge [9]. Additionally, they can be with several other systems and do not generate any problems in the environment.
Several genera of bacteria, including Deinococcus sp., Brucella sp., and Pseudomonas sp., are capable to decrease Cr(VI) to Cr(III) [13,14,15,16,17]. Chromium occurs naturally in its trivalent form (Cr3+), and at trace levels, it is essential to maintain fat, protein, and glucose metabolism in biological tissue [13, 18, 19]. To accomplish these restrictions, it is necessary to identify, quantify, and remove high levels of trace metals present in ecosystems and in food chains. Therefore, the accessibility of an active Cr(VI)-reducing bacterial strain with significant tolerance to Cr(VI) is a vital pre-requisite for evolving a bioremediation method intended for the decontamination of Cr(VI)-polluted wastewater. In this context, the present study aims to achieve the isolation of Cr(VI)-tolerant bacteria from chromium-polluted areas in Ranipet, Tamil Nadu, India, and also to characterize the features involved in Cr(VI) bioreduction by the bacterial isolates.
2 Materials and methods
2.1 Collection of sample and growth media preparation
A suitable bacterial growth media was designed by Murugavelh and Mohanty [20] for chromium removal, which is employed in this study with minor modifications. Two potent chromium tolerant bacteria (Bacillus thuringiensis (V45) and Staphylococcus capitis (S21)) strains used in this study were isolated from the chromium-contaminated sediment sites of Ranipet, Tamil Nadu, India, which is famous for its numerous tanneries and chemical treatment plants. The sampling site latitude and longitude are 12.93° N and 79.33° E. The sediment sample was collected from diverse locations in waterlogged areas and stored at − 4 °C. To identify and isolate minimum chromium tolerance bacterial isolate, we have used M9 medium as a base added with Cr(VI) concentration from 100 to 1000 μg/mL. To isolate potent chromium reducing bacteria, the M9 medium was used, consisting of the following: Na2HPO4, 6.0 gL−1; KH2PO4, 3.0 gL−1; NH4Cl, 1.0 gL−1; yeast extract, 2 gL−1; peptone, 10 gL−1; beef extract, 10 gL−1; MgSO4, 0.24 gL−1; glucose, 2 gL−1; and potassium dichromate (K2Cr2O7), with a pH of 7.4.
2.2 Isolation, culture condition, and identification of Cr(VI)-tolerant strains
Hexavalent Cr(VI)-tolerant strains were isolated from sediment samples by the following procedure. One gram of sediment was supplemented to 100 mL of sterile M9 liquid medium and incubated at 30 °C for 24 h in a shaking rocker operating at 150 rpm. After incubation, 1 mL of the bacterial in the medium suspension was transferred to another 100 mL of freshly prepared M9 medium; then it was incubated at 30 °C within a shaking rocker operating at 150 rpm. The mixed cultures that were applied to Cr(VI) tolerance was augmented using a sequence of allocations through slowly raising the Cr(VI) concentration from 100 to 1000 μg/mL. The augmented culture was sequentially diluted and plated on M9 medium with 1.5% agar containing different concentrations of Cr(VI) to obtain strains with a high concentration of Cr(VI) tolerance [21].
The two strains obtained with a high concentration tolerance were identified on the basis of the 16S rRNA sequence, determined by sequencing the polymerase chain reaction (PCR)-amplified 16S rRNA as described by Murugavelh and Mohanty [20], with minor modifications. Bacterial genomic DNA was separated from the isolate and purified using a DNA separation and purification kit (Genei, Bangalore, India). With the use of 0.1 μg of the genomic DNA as the template, the genes encoding for 16S rRNA were amplified. The primers used for PCR amplification F27 (5′-AGAGTTTGATCATGGCTCAG-3′) and R1492 (5′-GGCTACCTTGTTACGACTT-3′) [22] were synthesized at Bioserve Biotechnologies Pvt. Ltd., Hyderabad, India. Genes were amplified by denaturation at 94 °C for 1 min using 30 cycles, 56 °C for 1 min as annealing, and 72 °C for 2 min as an extension, followed by 72 °C for 7 min as the ending extension. The PCR product was sequenced by Bioserve Biotechnologies. The 16S rRNA gene sequence was examined with the use of a basic local alignment search tool, BLAST (NCBI, USA), for comparison software and phylogenic tree assembly by neighbor-joining algorithm PAUP 3.1.1 and phylogenetic analysis by means of parsimony [15].
2.3 Hexavalent Cr(VI) reduction and growth kinetics
The two potent Cr(VI)-reducing strains, i.e., Bacillus thuringiensis (V45) and Staphylococcus capitis (S21), were identified. Both were studied for chromium reduction using 250-mL Erlenmeyer flask with 100 mL of M9 medium supplemented with 50 μg/mL of initial Cr(VI) concentration as K2Cr2O7. The flasks were filled with 0.2 mL of freshly prepared inoculum and incubated at 30 °C with rocking at 150 rpm for 48 h. Samples were drawn after the incubation period and centrifuged at 10,000 rpm for 15 min. At first, the cell-free suspension was gathered; then, the decrease in Cr(VI) concentration was measured by means of the 1,5-diphenylcarbazid procedure [19, 23]. Each experiment was performed in triplicate, and standard deviations were used to interpret the mean values.
2.4 Industrial effluent preparation for the estimation of Cr(VI)
Before analysis, the dilution of the industrial effluent was carried out for 100 times, and then it was adjusted to pH 7. Considering the technique of Cr(VI) determination, the proper aliquots of the sample were utilized.
2.5 Estimation of reduced chromium by UV-Vis spectrophotometry
According to the technique developed by Nagaraj et al. [19], with slight alterations, the stock solution and reaction solutions were prepared. To prepare the stock standard solution, K2Cr2O7 was dried at 150 °C for 1 h and then placed in a desiccator. First, the transfer of a solution consisting of 0.2–10.0 μg (0.02–1.0 μg/L−1) of K2Cr2O7 into a sequence of 10-mL flasks was carried out; then, it was added with 2.0 mL of dapsone and 2.0 mL of hydroxylamine hydrochloride. After that, for completing the reaction, it was left for 5 min. Subsequently, 1 mL of N-(1-naphthyl)-ethylenediamine dihydrochloride (NEDA) was added, with thorough mixing after the addition of each solution up to calibration mark. The solutions were made up to a volume of 10 mL with the addition of distilled water. After waiting for the chromogenic reactions to take place, the absorbance of the complex reaction solution was detected at λ = 540 nm and compared with the calibration graph using UV-Vis spectrophotometry (Shimadzu, Kyoto, Japan).
2.6 Bacterial growth pattern study
The growth patterns of the hexavalent Cr(VI) tolerant strains V45 and S21 (above 150 μg/mL) were estimated by inoculating the strains in M9 medium with various initial concentrations of K2Cr2O7 ranging from 20 to 200 μg/mL and incubating at 30 °C for 96 h. Aliquots were taken for analysis at fixed intermissions to estimate the chromium reduction, and the growth pattern was studied. The measurement of the optical density was done at 600 nm using a UV-Vis spectrophotometer to estimate the changes in culture growth [24].
2.7 Factors influencing Cr(VI) reduction
The Cr(VI) reduction efficacy of isolates V45 and S21 was characterized by altering various environmental parameters. The temperature (25, 30, 35, 40, and 45 °C), initial pH values (5, 7, 9, 10, and 11), and initial Cr(VI) concentration (20–140 μg/mL) were studied. The aerobic batch culture mode was utilized to study the decrease of Cr(VI). Then, the Cr(VI) was added to the autoclaved minimal medium (100 mL) within 250-mL culture flasks; next, the mixture was inoculated with young bacterial culture. After that, it was incubated at 30 °C under shaking at 150 rpm for 96 h. Aliquots were withdrawn after the incubation period and centrifuged at 10,000 rpm for 15 min and then analyzed for the reduction of Cr(VI) as explained above. The activities of sugars (supplemented as 0.1% glucose, fructose, and lactose as the electron donor) and metal ions with the final concentrations of 10 μg/mL (Cu2+, Mn2+, Zn2+, Mo2+, Ni2+, Mg2+, Na+, and Ca2+) were investigated. Cr(VI) was added to the minimal medium (pH 7) within the culture flasks in a way to obtain a final concentration of 60 μg/mL, and it was incubated at 35 °C with rocking over a period of 96 h.
2.8 Fourier transform infrared spectroscopy analysis
Through a centrifugation at 10,000 rpm for 15 min, the bacterial biomass was attained; next, it was dried and powdered using a freeze-dryer. The powder obtained was used to make 2% potassium bromide (KBr) pellets ready for analysis. In this method, the sample is mixed properly with fine alkali halide powder; then it is pulverized finely and compressed using a hydraulic press into translucent sample disks at a pressure of 100 kg/cm2. After that, the disks were inserted into a Fourier transform infrared spectrometer (Perkin-Elmer Spectrum One FT-IR 4200). The FT-IR spectrum of the control biomass and the one used for the bioreduction of 60 μg/mL of Cr(VI) were attained in a single scan. Through referring to the described average values, the shifts in the FT-IR peaks were determined [25].
2.9 Scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDS)
The morphological examination of V45 and S21 bacteria with and without Cr(VI) was visualized by SEM photography, and the elemental composition was estimated using EDS. The bacterial cells were collected from the samples with and without Cr(VI) reaction broth by centrifugation at 4000×g for 30 min followed by drying. They were then prefixed according to the procedure of Thacker et al. [23] with gold coating. The gold-coated samples were observed with a JSM-6335F FE-SEM (JEOL).
2.10 Statistical analysis
All of the experiments carried out in this research were concluded in triplicate. The SPSS personal computer statistical package (V. 16, SPSS Inc., Chicago) was employed for the aim of statistically assessing the collected data.
3 Results and discussion
A total of 33 Cr(VI)-tolerant bacterial isolates from sediment samples were isolated and screened. The two bacteria strains enduring an upper concentration of Cr(VI) (100 μg/mL), V45 and S21, were selected for subsequent experiments. A total of 10 bacterial strains (V16, V32, V42, V45, V52, S12, S14, S19, S21, and S32) that were tolerant of higher concentrations of Cr(VI) were used for this study. The figure shows the corresponding 16S rRNA gene fragment amplification from genomic DNA, with the bands corresponding to the anticipated size of ~ 1.5 kp (Fig. S1) and the phylogeny of the 10 isolates shown in Fig. S2. The partial sequence of the 16S rRNA gene of the 10 isolates showed 99% sequence homology to Proteus mirabilis, Bacillus cereus, Pseudomonas mendocina, Bacillus thuringiensis, Bacillus cereus, Bacillus cereus, Pseudomonas sp., Bacillus megaterium, Staphylococcus capitis, and Pseudomonas fluorescens. These sequences were submitted to the NCBI GenBank with accession numbers of HM439512, HM439506, GQ910744, GU797777, GU797778, HM439510, HM439507, HM439508, HM439509, and HM439511, respectively. Notably, it can be seen from Fig. S3 that Bacillus thuringiensis (V45) and Staphylococcus capitis (S21) bacteria showed greater reductions of maximum Cr(VI) concentration compared to the other eight isolates. Therefore, these two strains were selected for further study. A 50 μg/mL Cr(VI) concentration was diminished by up to 77.23% and 86.0% with V45 and S21, respectively, within 48 h. Our findings are in good agreement with earlier study [26], showing that the Pseudomonas fluorescens LB 300 strain could decrease 61% of Cr(VI) over 28 h of observation with a primary Cr(VI) concentration of 3.14 μg/mL; 69% reduction was detected with a primary concentration of 20 μg/mL of Cr(VI), and 99.7% decrease was achieved with an original concentration of 11.25 μg/mL of Cr(VI). According to Elangovan et al. [27], who reported that the Bacillus sp. isolated from chromate-contaminated soil reduced Cr(VI) from 80 to 40 μg/mL after 42 h in a nutrient medium. Our results clearly demonstrate that V45 and S21 isolates have a higher capability for Cr(VI) reduction than the bacterial strains formerly described (Fig. S4). The two bacterial strains were capable of growth even at 200–1500 mg/L concentrations of Cr(VI). Both isolates exhibited a high or less comparable pattern of growth in Cr(VI)-containing broth, and a decline in biomass yield was observed with increasing concentrations of Cr(VI). The V45 isolate demonstrated a growth lag phase that was extended with the increase of the Cr(VI) concentration. Extension of the lag could be due to the adjustment of bacterial cells to a maximum concentration of Cr(VI) within the medium. Overall observation shows increasing Cr(VI) concentration decreases the multiplication rate of cells.
The decrease of Cr(VI) by V45 and S21 was assessed under altered temperatures (25, 30, 35, 40, and 45 °C). As can be observed in Fig. 1a, the most appropriate temperature for decreasing Cr(VI) was 35 °C for both strains; and the reduction rate was in the ranges of 42.15–85.23% for V45 and 52.31–89.45% for S21. Thus, the results obtained in the present paper are in complete agreement with those of previously conducted studies reporting that the optimum temperature for Cr(VI) reduction by Nesterenkonia sp. [28] and Providences sp. was 35 °C [23]. The strains V45 and S21 were found capable of decreasing Cr(VI) over a broad pH range of 5–11, as shown in Fig. 1b. The V45 and S21 strains had the capacity to reduce the Cr(VI) content by 86.42% and 97.34%, respectively, at pH 7 (Fig. 1b). Comparatively, strain V45 was efficient in reducing higher concentration of Cr(VI) than S21 strain. This might be due to the different metabolites produced by the strain. As reported by Wang et al. [29], at pH 6.5–8.5, chromium was decreased by an Enterobacter strain, and it was inhibited toughly below pH 5.0 and above pH 9.0. As confirmed by Thacker and Madamwar [30], the optimal growing of Ochrobactrum sp. and Cr(VI) reduction was obtained when the pH value was set to 7.
The effect of the time required for decreasing Cr(VI) was examined, and the obtained results are displayed in Fig. 2a (V45) and Fig. 2b (S21). As displayed in Fig. 2 a and b, Cr(VI) reduction ensued at the maximum concentration of 1400 μg/mL, and then complete Cr(VI) reduction was not achieved at this first time. However, Cr(VI) was totally reduced with lower concentrations after 96 h. The V45 and S21 isolates could reduce a maximum of 200 mg/L of Cr(VI) to zero in 36 and 24 h, respectively. At first, in case of Cr(VI) concentrations of 600, 1000, and 1400 mg/L, the V45 isolate achieved reductions to 14.2 (76.31%), 57.1 (42.89%), and 107.5 (23.20%) mg/L, respectively, within 96 h (Fig. 4e), whereas the S21 isolate was able to reduce 60, 100, and 140 μg/mL of Cr(VI) to 94.0 (84.29%), 50.8 (49.19%), and 98.2 (29.84%) μg/mL, respectively, within 96 h. The impacts of changed Cr(VI) concentrations demonstrated that the activity of Cr(VI) could be decreased by the isolates with the use of an enzymatic reaction; however, the increase of the chromium level decreased the Cr(VI) reduction rate. According to Han et al. [31], up to 65% Cr(VI) was removed by Bacillus sphaericus within 8 h from the initial concentration of 100 μg/mL, and the Cr(VI) was completely reduced after 150 h. An Arthrobacter sp. exposed to tannery waste-polluted soil for a long time was isolated and examined for its capability of decreasing Cr(VI); findings showed that it could decrease Cr(VI) at a concentration of 30 μg/mL within 46-h incubation. On the other hand, at the concentration of 100 μg/mL, Cr(VI) did not decrease [32]. Both of the strains V45 and S21 showed the reductions up to 60.0% and 76.78%, respectively, for a concentration of 60 μg/mL of Cr(VI) after 36 h. These values increased to more than 76 and 84.29%, respectively, within 96 h, as observed in this experiment. This study obviously shows the isolates adaptation to high chromium levels. In all the experiments, bioreduction was observed throughout the exponential growth phase. The organisms were obtained from diversified locations and belonged to different genera or strains. They showed exceptional adaptation to the adjacent functions and exhibited bioreduction characteristics.
In literature, it has formerly been suggested that Cr(VI)-reducing microorganisms might use various organic compounds as electron donors and inorganic catalysts such as metal ions for Cr(VI) reduction [33, 34]. In this study, the chromate reduction activity of V45 and S21 isolates was determined in the presence of (1 g/L) electron donors (glucose, fructose, and lactose; Fig. 3) and (10 μg/mL) metal ions (Cu2+, Mn2+, Zn2+, Mo2+, Ni2+, Mg2+, Na+, and Ca2+) (Fig. 4). Among the electron donors tested, 1 g/L of glucose enhanced the Cr(VI) reduction capability of V45 and S21 isolates up to 82.70 and 90.57%, respectively, at 60 μg/mL of Cr(VI) in 96 h (Fig. 3). In contrast, the supplement of fructose and lactose did not affect the decrease of Cr(VI) by either of the isolates (Fig. 5a). In Bacillus sp., an increase in the Cr(VI) reduction was observed in cases where it was supplemented with glucose [24]. Therefore, our findings are similar to the reports of Wang et al. [33], in which glucose promoted Cr(VI) decrease by Penicillium sp.
In addition, the way other metal ion cations affect the Cr(VI) decrease was investigated with the use of the V45 and S21 isolates. Based on Fig. 4, Cu2+ and Na+ marginally increased the rate of Cr(VI) decrease, while Mn2+, Ca2+, Mo2+, Zn2+, Mg2+, and Ni2+ prevented the Cr(VI) decrease by the V45 isolate. The decrease of Cr(VI) by the S21 isolate was augmented in the presence of Cu2+, Mn2+, and Na+, whereas Zn2+, Mo2+, Ni2+, Mg2+, and Ca2+ inhibited Cr(VI) reduction (Fig. 4). According to the study by Camargo et al. [35] stimulatory effect of Cu2+ and Mn2+ on Cr(VI) reduction activity has been also reported for Cr(VI)- reduction by Bacillus sp. ES 29 cell free extract. Monovalent Na+ also increased Cr(VI) reduction by Bacillus sphaericus GIDM [16]. The decrease of chromate by B. sphaericus was inhibited due to the existence of metal ions such as Co2+ and Ni2+ at both high (100 μg/mL) and low (20 μg/mL) concentrations. This is not clear how Cu2+ interacts with other metals. However, in several reductase enzymes, the Cu2+ is present as a prosthetic group. The Cu2+ generally plays two different roles: an electron mobility shield or an electron redox center; in addition, in certain circumstances, it acts as a vehicle for electrons among protein subunits [36]. When oxygen is present, the decrease of bacterial Cr(VI) takes place typically as a route involving two or three steps; in this condition, Cr(VI) primarily decreases to the short-lived intermediates Cr(V) and/or Cr(IV) before the final decrease to the thermodynamically stable end product, namely, Cr(III). On the other hand, it is not clear whether the decrease of Cr(V) to Cr(IV) and Cr(IV) to Cr(III) is an enzyme-mediated process or totally impulsive. According to Appenroth et al. [37], during the process of Cr(VI) decrease, NADH, NADPH, and electrons from the endogenous reserve can act as electron donors. Moreover, the exact way through which electrons are moved from glucose towards Cr(VI) has not been clarified yet. The representative soil samples were collected in and around tannery industries for physicochemical analysis like pH (8.3 ± 0.22), EC (19.20 ± 0.45mS/cm), nitrogen (59.0 ± 0.12), sodium (250 ± 0.61), chloride (68 ± 0.43), moisture content (12.82 ± 1.24), phosphorus (5.9 ± 0.38), and potassium (186.0 ± 1.48), and garden soil is used as a control.
The FT-IR data suggest that metabolic alterations occur among cells grown under Cr(VI) concentrations in minimal medium in sub-aerobic conditions. The FT-IR spectra of the control biomass and Cr(VI)-treated biomass of V45 and S21 isolates are displayed in Fig 5a and b, respectively. As can be seen in Fig. 5a, in the sample treated, a peak exists at 1740 cm−1, which may be attributed to the C═O stretching of carboxyl groups [38]. As indicated by the peak consolidation at 1650 cm−1, which is related to the C═O chelate stretching, carboxyl groups have effect on chromium binding through bioreduction, which are in agreement with Park et al. [11]. The absorption peak at 1650 cm−1 can be attributed to the amide I and amide II bands of the amide bond of protein peptide bonds [39]. The absorption peaks in the range of 1530–1650 cm−1 was corresponding to –NH bending; as a result, it can be said that the amino groups may have played a role in the bioreduction of Cr(VI) [11]. When a peak exists at 1558 cm−1, it may point to the existence of amide II, which is due to the NH deformation mode conjugated to a C═N group [40]. As amino groups are the chief constituents of the cell wall, changes to bending may be because of the chromium binding [9]. The strengthening of peak at 1400 cm−1 is a characteristic peak of symmetric vibrational COO− frequencies of terminal amino acids. Such alteration is in agreement with the Han et al. [37] findings. The modest absorption peak at 1050 cm−1 can be attributed to the –CN stretching vibration of the protein fractions [40].
The Cr(VI) treatment leads to various alterations in the FT-IR spectrum of the S21 isolate (Fig. 5b). The development of minor peaks among 3200 and 3600 cm−1 can be because of the overlying of –OH and –NH stretching. Such result agrees with those of Deng and Ting [41], who stated that it is not easy to explore the reason for the alterations in the functional groups among these areas. The loss of the peak at 1240 cm−1 represents the P═O band of polysaccharides [42]. The vanishing of peaks at 2350 and 2790 cm−1 is accredited to the O–H and N–C–O stretching, respectively. Parallel alterations in the FT-IR spectrum as logged above were observed in all the organisms when their treatment using chromium represents a similar bioreduction mechanism in all of the cases. Only minor alterations of the other absorption frequencies were observed, though it was not easy to understand the way these absorption peaks were corresponding to the bioreduction of Cr(VI). The biomass oxidation in the Cr(VI) bioreduction may lead to a few variations in the absorption frequencies of a number of functional groups that are present within the biomass.
The SEM image of V45 and S21 cell morphology was observed after the treatment of the bacteria for 72 h with and without 50 μg/mL of K2Cr2O7 (Figs.6 a, b, c, d, and e). Figure 6 a (control) and b (with Cr(VI)) showed that the V45 cells that appear as stretched rods with projections on the cell surfaces along with the production of more capsular material, revealing an increase in the thickness of the cell walls in the occurrence of Cr(VI). Figure 6 c shows the occurrence of elemental chromium. Other elements might have emerged from the medium, surface coating, and sample base. Figure 6 d (control) and e (with Cr(VI)) showed that the cell morphology of S21 with slight projections emerging from a spherical shape. In the absence of K2Cr2O7, the two cells exhibited no change in shape and appeared as rods and cocci with smooth surfaces (Figs. 6 d and e). Figure 6 f shows the occurrence of elemental chromium obtained from the S21 cell surface. Earlier Şahin Y and Öztürk [43] have studied the biosorption of Cr(VI) ions from aqueous solutions on dried vegetative cell and spore-crystal mixture of Bacillus thuringiensis var. thuringiensis. This study well supported to our results that different parameters showed that vegetative cell and spore-crystal mixture of B. thuringiensis var. thuringiensis strain are excellent adsorbents for Cr(VI) ions.
a–f SEM images and EDS data of B. thuringiensis strain V45 and S. capitis strain S21. (a) B. thuringiensis strain V45 cells grown in the presence of 1000 μg/ml of K2CrO4 for 48 h Scale bar: 1 (arrow indicates binary cell fission) (b) Scale bars: 5 μm. (c) EDS B. thuringiensis strain V45 (d) S. capitis strain S21 cells grown in the presence of 1000 μg/ml of K2CrO4 for 48 h Scale bar: 1 (arrow indicates binary cell fission) (e) Scale bars: 5 μm. (f) EDS S. capitis
According to Joutey et al. [44], the mechanism of chromium reduction by B. thuringiensis and S. capitis: most of the chromium reduction mechanism by bacteria was started with Cr(VI) and ended with Cr(III) as a reduction via several enzyme-mediated mechanism according to the environmental conditions. Enzyme-mediated reduction mechanism works in two-phase aerobic, and anaerobic phase, aerobic phase NAD(P) H, and endogenous e-reserves are implicated as electron donors for Cr(VI) reduction by reductases like ChrR and YieF. Anaerobic phase both soluble (SR) and membrane-associated (MR) enzymes mediates Cr(VI) reduction. Metabolites of some anaerobes such as H2S (produced by SRB and Fe(II) by IRB) are effective Cr(VI) reductants.
4 Conclusion
The current study demonstrated the isolation of organisms from effluents and sediments of Cr(VI)-contaminated waste. The bioreduction of more than 90.75% of 60 μg/mL of Cr(VI) was achieved from a synthetic effluent during 96 h, and it was indicated that the highest degree of bioreduction occurred through the exponential growth stage. The present study isolated the most active organisms (Bacillus thuringiensis and Staphylococcus capitis) from this study area for the first time. Various optimization parameters like temperature, pH, and Cr concentration were investigated as significant environmental factors that can regulate the remediation strategies for those ecosystems that are polluted with anthropogenic or natural Cr(VI). The results of the analyses carried out using FTIR demonstrated that amino and carboxyl groups had impacts on detoxification of Cr(VI). Chemical variations were conducted on biomass in concentration of the amino and carboxyl groups for the aim of examining their effects in the detoxification of Cr(VI). The SEM images confirmed the accumulation of metal around the cells, and EDS showed the occurrence of metallic chromium. The isolates described in this work showed a promising ability to reduce Cr(VI), and their features can help in the bioremediation process of the Cr(VI) ion. This method offers a higher cost-effectiveness in comparison with the chemical/physical methods conventionally applied to treating the Cr(VI)-contaminated environments.
References
Oliveira H (2012) Chromium as an environmental pollutant: insights on induced plant toxicity. Journal of Botany 2012:375843, 8 pages. https://doi.org/10.1155/2012/375843
Stanin FT (2005) The transport and fate of chromium (VI) in the environment. In: J Guertin, JA Jacobs, CP Avakian (Eds) Chromium(VI) Handbook, CRC Press, Florida, pp. 165–214
Mandal BK, Vankayala R, Uday Kumar L (2011) Speciation of chromium in soil and sludge in the surrounding tannery region, Ranipet, Tamil Nadu. ISRN Toxicol 2011:697980
Bianchi V, Levis AG (1987) Recent advances in chromium genotoxicity. Toxicol Environ Chem 15(1–2):1–23
Mishra S, Bharagava RN (2016) Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J Environ Sci Health Part C 34(1):1–32
Lu YZ, Chen GJ, Bai YN, Fu L, Qin LP, Zeng RJ (2018) Chromium isotope fractionation during Cr (VI) reduction in a methane-based hollow-fiber membrane biofilm reactor. Water Res 130:263–270
Mauri R, Shinnar R, d'Amore M, Giordano P, Volpe A (2001) Solvent extraction of chromium and cadmium from contaminated soils. AICHE J 47(2):509–512
Manikandan V, Balamuralikrishnan B, Palanivel V, Hesam K, Arumugam V, Shreeshivadasan C, Lee CT, Jayanthi P (2021) Fabrication of nanocomposites mediated from aluminium nanoparticles/Moringa oleifera gum activated carbon for effective photocatalytic removal of nitrate and phosphate in aqueous solution. J Clean Prod 281:124553.
Bai RS, Abraham TE (2003) Studies on chromium (VI) adsorption–desorption using immobilized fungal biomass. Bioresour Technol 87(1):17–26
Rengaraj S, Joo CK, Kim Y, Yi J (2003) Kinetics of removal of chromium from water and electronic process wastewater by ion exchange resins: 1200H, 1500H and IRN97H. J Hazard Mater 102(2–3):257–275
Park D, Yun YS, Park JM (2005) Studies on hexavalent chromium biosorption by chemically-treated biomass of Ecklonia sp. Chemosphere 60(10):1356–1364
Ucun H, Bayhan YK, Kaya Y, Cakici A, Algur OF (2002) Biosorption of chromium (VI) from aqueous solution by cone biomass of Pinus sylvestris. Bioresour Technol 85(2):155–158
Fredrickson JK, Kostandarithes HM, Li SW, Plymale AE, Daly MJ (2000) Reduction of Fe (III), Cr (VI), U (VI), and Tc (VII) by Deinococcus radiodurans R1. Appl Environ Microbial 66(5):2006–2011
Thacker U, Parikh R, Shouche Y, Madamwar D (2007) Reduction of chromate by cell-free extract of Brucella sp. isolated from Cr (VI) contaminated sites. Bioresour Technol 98(8):1541–1547
Song H, Liu Y, Xu W, Zeng G, Aibibu N, Xu L, Chen B (2009) Simultaneous Cr (VI) reduction and phenol degradation in pure cultures of Pseudomonas aeruginosa CCTCC AB91095. Bioresour Technol 100(21):5079–5084
Desai C, Jain K, Madamwar D (2008a) Evaluation of in vitro Cr (VI) reduction potential in cytosolic extracts of three indigenous Bacillus sp. isolated from Cr (VI) polluted industrial landfill. Bioresour Technol 99(14):6059–6069
Desai C, Jain K, Madamwar D (2008b) Hexavalent chromate reductase activity in cytosolic fractions of Pseudomonas sp. G1DM21 isolated from Cr (VI) contaminated industrial landfill. Process Biochem 43(7):713–721
Lowe KL, Straube W, Little B, Jones Meehan J (2003) Aerobic and anaerobic reduction of Cr (VI) by Shewanella oneidensis effects of cationic metals, sorbing agents and mixed microbial cultures. Acta Biotechnol 23(2–3):161–178
Nagaraj P, Aradhana N, Shivakumar A, Shrestha AK, Gowda KA (2009) Spectrophotometric method for the determination of chromium (VI) in water samples. Environ Monit Assess 157(1–4):575–582
Murugavelh S, Mohanty K (2013) Isolation, identification and characterization of Cr (VI) reducing Bacillus cereus from chromium contaminated soil. Chem Eng J 230:1–9
Morales-Barrera L, de María G-JF, Ortiz-Moreno A, Villegas-Garrido TL, Sandoval-Cabrera A, Hernández-Rodríguez CH, Cristiani-Urbina E (2008) Isolation, identification and characterization of a Hypocrea tawa strain with high Cr (VI) reduction potential. Biochem Eng J 40(2):284–292
Heuer H, Krsek M, Baker P, Smalla K, Wellington EM (1997) Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol 63(8):3233–3241
Thacker U, Parikh R, Shouche Y, Madamwar D (2006) Hexavalent chromium reduction by Providencia sp. Process Biochem 41(6):1332–1337
Pal A, Paul AK (2004) Aerobic chromate reduction by chromium-resistant bacteria isolated from serpentine soil. Microbiol Res 159(4):347–354
Silverstein RM, Bassler GC (1962) Spectrometric identification of organic compounds. J Chem Educ 39(11):546
DeLeo PC, Ehrlich HL (1994) Reduction of hexavalent chromium by Pseudomonas fluorescens LB300 in batch and continuous cultures. Appl Microbiol Biotechnol 40(5):756–759
Elangovan R, Abhipsa S, Rohit B, Ligy P, Chandraraj K (2006) Reduction of Cr (VI) by a Bacillus sp. Biotechnol Lett 28(4):247–252
Amoozegar MA, Ghasemi A, Razavi MR, Naddaf S (2007) Evaluation of hexavalent chromium reduction by chromate-resistant moderately halophile. Nesterenkonia sp. strain MF2. Process Biochem 42(10):1475–1479
Wang PC, Mori T, Toda K, Ohtake H (1990) Membrane-associated chromate reductase activity from Enterobacter cloacae. J Bacteriol 172(3):1670–1672
Thacker U, Madamwar D (2005) Reduction of toxic chromium and partial localization of chromium reductase activity in bacterial isolate DM1. World J Microbiol Biotechnol 21(6–7):891–899
Han X, Wong YS, Wong MH, Tam NFY (2007) Biosorption and bioreduction of Cr (VI) by a microalgal isolate Chlorella miniata. J Hazard Mater 146(1–2):65–72
Megharaj M, Avudainayagam S, Naidu R (2003) Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr Microbiol 47(1):0051–0054
Wang YT, Xiao C (1995) Factors affecting hexavalent chromium reduction in pure cultures of bacteria. Water Res 29(11):2467–2474
Guha H, Jayachandran K, Maurrasse F (2001) Kinetics of chromium (VI) reduction by a type strain Shewanella alga under different growth conditions. Environ Pollut 115(2):209–218
Camargo FAO, Bento FM, Okeke BC, Frankenberger WT (2003) Chromate reduction by chromium-resistant bacteria isolated from soils contaminated with dichromate. J Environ Qual 32(4):1228–1233
Abe F, Miura T, Nagahama T, Inoue A, Usami R, Horikoshi K (2001) Isolation of highly copper-tolerant yeast, Cryptococcus sp., from the Japan Trench and the induction of superoxide dismutase activity by cu 2+. Biotechnol Lett 23(24):2027–2034
Appenroth KJ, Bischoff M, Gabryś H, Stoeckel J, Swartz HM, Walczak T, Winnefeld K (2000) Kinetics of chromium (V) formation and reduction in fronds of the duckweed Spirodela polyrhiza—a low frequency EPR study. J Inorg Biochem 78(3):235–242
Yun YS, Park D, Park JM, Volesky B (2001) Biosorption of trivalent chromium on the brown seaweed biomass. Environmental science & technology 35(21):4353–4358
Yee N, Benning LG, Phoenix VR, Ferris FG (2004) Characterization of metal− cyanobacteria sorption reactions: a combined macroscopic and infrared spectroscopic investigation. Environmental science & technology 38(3):775–782
Kapoor A, Viraraghavan T (1997) Heavy metal biosorption sites in Aspergillus niger. Bioresour Technol 61(3):221–227
Deng S, Ting YP (2005) Characterization of PEI-modified biomass and biosorption of Cu (II), Pb (II) and Ni (II). Water Res 39(10):2167–2177
Benning LG, Phoenix VR, Yee N, Tobin MJ (2004) Molecular characterization of cyanobacterial silicification using synchrotron infrared micro-spectroscopy. Geochim Cosmochim Acta 68(4):729–741
Şahin Y, Öztürk A (2005) Biosorption of chromium (VI) ions from aqueous solution by the bacterium Bacillus thuringiensis. Process Biochem 40(5):1895–1901
Joutey NT, Sayel H, Bahafid W, El Ghachtouli N (2015) Mechanisms of hexavalent chromium resistance and removal by microorganisms. In: Reviews of Environmental Contamination and Toxicology, vol 233. Springer, Cham, pp 45–69
Acknowledgements
Financial support rendered to Palanivel Velmurugan through RUSA 2.0 scheme in the form of Senior Postdoctoral fellowship [Grant No. F. 24-51/2014-U, Policy (TN Multi-Gen), Department of Education, Government of India] is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Balamuralikrishnan Balasubramanian is equal contributor as first author
Supplementary information
ESM 1
(DOC 885 kb)
Rights and permissions
About this article
Cite this article
Suresh, G., Balasubramanian, B., Ravichandran, N. et al. Bioremediation of hexavalent chromium-contaminated wastewater by Bacillus thuringiensis and Staphylococcus capitis isolated from tannery sediment. Biomass Conv. Bioref. 11, 383–391 (2021). https://doi.org/10.1007/s13399-020-01259-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01259-y