Abstract
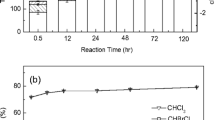
The catalytic hydrodechlorination of 2,4-dichlorophenol (2,4-DCP) as a model pollutant has been conducted for evaluation of optimum base in laboratory scale cocurrent downflow contactor reactor (CDCR). 2,4-DCP was completely dechlorinated with an initial concentration of 3.0 and 6.0 mmol, at 303 K temperature and 0.1 MPa pressure. Hydrogen flow rate was remained at 0.3 L/min, and catalyst loading of 0.2 g/L of 5 % Palladium/carbon (Pd/C) catalyst was used. Different bases like triethylamine \((\hbox {N(CH}_{2}\hbox {CH}_{3})_{3})\), sodium acetate trihydrate \((\hbox {CH}_{3}\hbox {COONa}\cdot 3\hbox {H}_{2}\hbox {O})\), sodium hydroxide (NaOH), ammonium hydroxide \((\hbox {NH}_{4}\hbox {OH})\) and potassium hydroxide (KOH) were used for evaluation of optimum base. The same reaction was studied without base, and it was observed that more reaction time was required for hydrodechlorination of 2,4-DCP as compared to the presence of a base as production of HCl decreased the rate of reaction. During the hydrodechlorination reaction, 4-chlorophenol (4-CP) was also formed as an intermediate product along with 2-chlorophenol (2-CP). Significant amount of 4-CP produced when no alkali or weak alkalis like \(\hbox {N(CH}_{2}\hbox {CH}_{3})_{3}\), \(\hbox {CH}_{3}\hbox {COONa}\cdot 3\hbox {H}_{2}\hbox {O}\) were used. No inhibitory effects of \(\hbox {N(CH}_{2}\hbox {CH}_{3})_{3}\) were observed during hydrodechlorination reaction of 2,4-DCP in CDCR. The addition of strong inorganic base helped with dechlorination of 2,4-DCP in predominantly fast reaction, and rate of neutralization using different bases was observed and found that the KOH had highest rate of reaction and \(\hbox {CH}_{3}\hbox {COONa}\cdot 3\hbox {H}_{2}\hbox {O}\) had lowest, whereas NaOH, \(\hbox {NH}_{4}\hbox {OH}\) and \(\hbox {N(CH}_{2}\hbox {CH}_{3})_{3}\) had intermediate reaction time. The presence of strong base kept pH in the basic region after neutralization of all HCl formed during the reaction.
Similar content being viewed by others
References
Olaniran, A.O.; Igbinosa, E.O.: Chlorophenols and other related derivatives of environmental concern: properties, distribution and microbial degradation processes. Chemosphere. 83, 1297–1306 (2011)
US EPA: ATSDR—Priority List of Hazardous Substances. http://www.atsdr.cdc.gov/SPL/index.html (2011)
Nomngongo, P.N.; Catherine Ngila, J.; Msagati, T.A.M.; Gumbi, B.P.; Iwuoha, E.I.: Determination of selected persistent organic pollutants in wastewater from landfill leachates, using an amperometric biosensor. Phys. Chem. Earth 50–52, 252–261 (2012)
Ozkaya, B.: Chlorophenols in leachates originating from different landfills and aerobic composting plants. J. Hazard. Mater. 124, 107–112 (2005)
Czaplicka, M.: Sources and transformations of chlorophenols in the natural environment. Sci. Total Environ. 322, 21–39 (2004)
WHO: Guidelines for drinking-water quality. Health criteria and other supporting information, vol. 2, 2nd edn. World Health Organization, Geneva, 828–836 (1996)
Kantarci, N.; Borak, F.; Ulgen, K.O.: Bubble column reactors. Process Biochem. 40, 2263–2283 (2005)
Winterbottom, J.M.; Khan, Z.; Boyes, A.P.; Raymahasay, S.: Catalytic hydrogenation in a packed bed bubble column reactor. Catal. Today 48, 221–228 (1999)
Ochuma, I.J.; Fishwick, R.P.; Wood, J.; Winterbottom, J.M.: Photocatalytic oxidation of 2,4,6-trichlorophenol in water using a cocurrent downflow contactor reactor (CDCR). J. Hazard. Mater. 144, 627–633 (2007)
Ochuma, I.J.; Fishwick, R.P.; Wood, J.; Winterbottom, J.M.: Optimisation of degradation conditions of 1,8-diazabicyclo[5.4.0]undec-7-ene in water and reaction kinetics analysis using a cocurrent downflow contactor photocatalytic reactor. Appl. Catal. B Environ. 73, 259–268 (2007)
Yuan, G.; Keane, M.A.: Catalyst deactivation during the liquid phase hydrodechlorination of 2,4-dichlorophenol over supported Pd: influence of the support. Catal. Today 88, 27–36 (2003)
Yuan, G.; Keane, M.A.: Liquid phase catalytic hydrodechlorination of 2, 4-dichlorophenol over carbon supported palladium? an evaluation of transport limitations. Chem. Eng. Sci. 58, 257–267 (2003)
Yuan, G.; Keane, M.A.: Liquid phase catalytic hydrodechlorination of chlorophenols at 273 K. Catal. Commun. 4, 195–201 (2003)
Shin, E.J.; Keane, M.A.: Gas phase catalytic hydrodechlorination of chlorophenols using a supported nickel catalyst. Appl. Catal. B Environ. 18, 241–250 (1998)
Gómez-Quero, S.; Cárdenas-Lizana, F.; Keane, M.A.: Unique selectivity in the hydrodechlorination of 2,4-dichlorophenol over hematite-supported Au. J. Catal. 303, 41–49 (2013)
Yuan, G.; Keane, M.A.: Liquid phase hydrodechlorination of chlorophenols over Pd/C and Pd/Al 2O3: a consideration of HCl/catalyst interactions and solution pH effects. Appl. Catal. B Environ. 52, 301–314 (2004)
Ma, X.; Zhou, S.; Yang, C.; Liu, S.; Bi, X.; Xia, C.: The influence of triethylamine on the hydrodechlorination reactivity of chlorophenols over Raney Ni catalyst. Catal. Commun. 12, 282–285 (2010)
Jin, Z.; Yu, C.; Wang, X.; Wan, Y.; Li, D.; Lu, G.: Liquid phase hydrodechlorination of chlorophenols at lower temperature on a novel Pd catalyst. J. Hazard. Mater. 186, 1726–32 (2011)
Yuan, G.: Role of base addition in the liquid-phase hydrodechlorination of 2,4-dichlorophenol over Pd/Al2O3 and Pd/C. J. Catal. 225, 510–522 (2004)
Felis, V.; De Bellefon, C.; Fouilloux, P.; Schweich, D.: Hydrodechlorination and hydrodearomatisation of monoaromatic chlorophenols into cyclohexanol on Ru/C catalysts applied to water depollution? influence of the basic solvent and kinetics of the reactions. Appl. Catal. B Environ. 20, 91–100 (1999)
Rehman, A.; Nawaz, M.; Chughtai, A.; Arif, M.: Catalytic hydrodechlorination of 2, 4-dichlorophenol using co-current down flow contactor reactor. Pak. J. Sci. Ind. Res. Ser. A Phys. Sci. 58, 1–7 (2015)
Sahinkaya, E.; Dilek, F.B.: Biodegradation of 4-CP and 2,4-DCP mixture in a rotating biological contactor (RBC). Biochem. Eng. J. 31, 141–147 (2006)
Jia, J.; Zhang, S.; Wang, P.; Wang, H.: Degradation of high concentration 2,4-dichlorophenol by simultaneous photocatalytic-enzymatic process using TiO2/UV and laccase. J. Hazard. Mater. 205–206, 150–155 (2012)
Haggblom, M.M.; Young, L.Y.: Chlorophenol degradation coupled to sulfate reduction. Appl. Environ. Microbiol. 56, 3255–3260 (1990)
Sahinkaya, E.; Dilek, F.B.: Biodegradation kinetics of 2,4-dichlorophenol by acclimated mixed cultures. J. Biotechnol. 127, 716–726 (2007)
Zhang, C.; Zhou, M.; Ren, G.; Yu, X.; Ma, L.; Yang, J.; Yu, F.: Heterogeneous electro-Fenton using modified iron-carbon as catalyst for 2,4-dichlorophenol degradation: influence factors, mechanism and degradation pathway. Water Res. 70, 414–424 (2015)
Petroutsos, D.; Katapodis, P.; Samiotaki, M.; Panayotou, G.; Kekos, D.: Detoxification of 2,4-dichlorophenol by the marine microalga Tetraselmis marina. Phytochemistry 69, 707–714 (2008)
Chen, H.; Zhang, Z.; Yang, Z.; Yang, Q.; Li, B.; Bai, Z.: Heterogeneous fenton-like catalytic degradation of 2,4-dichlorophenoxyacetic acid in water with FeS. Chem. Eng. J. 273, 481–489 (2015)
Hoke, J.B.; Gramiccloni, G.A.; Balko, E.N.: Catalytic hydrodechlorinatian of chlorophenols. Appl. Catal. B Environ. 1, 285–296 (1992)
Ribeiro, F.; Gerken, C.; Somorjai, G.; Kellner, C.: Turnover rate and kinetic mechanism for the reaction of hydrodechlorination of 1, 1-dichlorotetrafluoroethane (CF 3-CFCl 2) over a polycrystalline Pd foil. Catal. Lett. 45, 149–153 (1997)
Jin, Z.; Wang, X.; Wang, S.; Li, D.; Lu, G.: The effect of triethylamine on the hydrodechlorination of chlorophenols on Pd/C at low temperature. Catal. Commun. 10, 2027–2030 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rehman, A., Chughtai, A., Ijaz, A. et al. Evaluation of Base for Catalytic Hydrodechlorination of 2,4-Dichlorophenol in Cocurrent Downflow Contactor Reactor. Arab J Sci Eng 42, 1419–1425 (2017). https://doi.org/10.1007/s13369-016-2300-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-016-2300-6