Abstract
We report the mass spectrometric detection of hydrogenated gold clusters ionized by electron transfer and proton transfer. The cations appear after the pickup of hydrogen molecules and gold atoms by helium nanodroplets (HNDs) near zero K and subsequent exposure to electron impact. We focus on the size distributions of the gold cluster cations and their hydrogen content, the electron energy dependence of the ion yield, patterns of hydrogenated gold cluster cation stability, and the presence of “magic” clusters. Ab initio molecular orbital calculations were performed to provide insight into ionization energies and proton affinities of gold clusters as well as into molecular hydrogen affinities of the ionized and protonated gold cluster cations.

Similar content being viewed by others
Introduction
Gold, precious in so many other ways, is at most only moderately effective as a catalyst, at least as a clean bulk metal, when compared to group VIII to X metals including platinum for example, its neighbor on the periodic table. As a hydrogenation catalyst, pure bulk gold has been found to have only a weak affinity for molecular hydrogen (unless dispersed and supported on a metal oxide) [1, 2]. Also, there appears to be no direct evidence that molecular hydrogen chemisorbs by dissociation on bulk gold at room temperature and below.
But gold behaves differently as very small clusters of atoms [3,4,5,6,7,8]. The chemical nature of small aggregates of gold has been studied extensively in recent decades [9,10,11] and has led to the development of, for example, gold-based catalysts [12, 13]. More specifically, the history of studies on gold-hydrogen complexes goes back at least a century [14]. Since then, numerous studies of complexes of gold and hydrogen have been carried out [15,16,17]. Computations have shown that Au2 and Au3 bind one and even two molecules of hydrogen, the first with binding energies (De) of 0.55 and 0.71 eV, respectively [18]. However, the computations also predict the presence of a substantial energy barrier for the dissociation of adsorbed hydrogen, 1.10 and 0.59 eV, respectively. Other calculations using density functional theory, as well as infrared spectroscopy experiments in solid hydrogen, have characterized AuH, AuH2, (H2)AuH, and (H2)AuH3 [19, 20] and the decomposition of AuH2 by the release of H2 [20]. Sugawara et al. studied reactions of small gold cluster cations Aun+ (n = 1–12) with molecular hydrogen in an FT-ICR mass spectrometer and did not observe any reaction products [21]. However, mixed cluster ions of the form AunHx+ (n < 8) are efficiently formed via laser ablation of a gold rod in an atmosphere of a hydrogen (5.3%)/helium mixture. Pronounced intensity anomalies of these cations as a function of the number of attached hydrogen atoms, x, have been reported [21].
Here, we apply a very low temperature technique with which we can encourage both Au atoms to cluster and molecular hydrogen to adsorb on these clusters within a superfluid helium environment provided by helium droplets [22,23,24,25,26]. A beam of nanodroplets of helium is seeded with molecules of hydrogen and atoms of gold and these are allowed to interact before electron impact ionization of the droplets. In this way, clusters of gold and hydrogen are allowed to form and are then exposed to electron and proton transfer reactions that produce positive ions that ultimately are detected mass spectrometrically. The mass spectra provide the stoichiometry of the hydrogenated gold cluster cations as a function of cluster size and, indirectly, insight into the precursor neutral hydrogenated gold clusters. Furthermore, with molecular orbital calculations, we explore the energetics of gold clusters losing electrons or gaining protons as well as the structures and stabilities of the hydrogenated gold cluster cations that are observed to “magically” predominate in the mass spectra.
Experimental
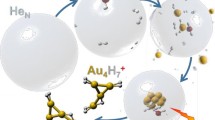
The experimental apparatus is described in detail elsewhere [22, 27,28,29], but an overview of the processes involved can be found in Figure 1. He nanodroplets were produced via supersonic expansion of pre-cooled gaseous He (Messer, 99.9999% purity) under a pressure of 2.25 MPa through a 5-μm diameter nozzle cooled to 9.55 K. The mean size of the produced droplets is estimated to be 106 He atoms [30, 31] and their velocity is approximately 260 m/s. The helium beam passed through a 0.8-mm diameter skimmer and entered a pickup region where hydrogen (Messer Austria GmbH, purity 99.999%) was introduced via a needle valve. Gold vapor was produced from solid gold heated with 118 W, which gives a temperature of at least 950 °C, in an oven similar to the one reported by Feng et al [26] that is located another 115 mm downstream. The vapor pressures in the two pickup cells are on the order of 10−6 mbar. The doped droplets underwent ionization in a Nier-type ion source with electron kinetic energies of 85 eV for positive ion formation. The dopants were ionized through different processes depending on the polarity [32, 33]. The ionized complexes were then driven through a set of Einzel lenses into the extraction region of a commercial, reflectron time-of-flight mass spectrometer (Tofwerk AG, model HTOF) where spectra of the signal intensity versus mass per charge were obtained. The spectra were evaluated in the custom software IsotopeFit with which overlaps were deconvoluted, background signals were subtracted, and mass peaks were fitted [34].
Overview of the formation of gold/hydrogen clusters from doped He nanodroplets. Neutral helium droplets containing millions of atoms capture gas phase H2 and Au atoms in sequential pickup chambers and condense into mixed, physisorbed aggregates. The doped droplets are ionized by electron impact (EI) which leads to the formation of charged AunHx+ clusters that are analyzed by mass spectrometry
Theory
We have investigated structures and properties of pure and hydrogenated gold clusters with ab initio calculations using second-order Møller-Plesset (MP2) perturbation theory. In order to find the most energetically preferred structure for each cluster size (each unique combination of Au and H atoms), we optimized the structures for any different starting geometries at the MP2/def2-SVP level. We then further optimized the most stable candidates at a higher MP2/def2-TZVP level. Core potentials (as defined in the respective basis sets) were utilized for the Au atoms to include relativistic corrections and to speed up the calculations. A vibrational frequency analysis was performed to ensure that proper minima were achieved in the structure optimizations and to calculate the zero-point energy corrections that are included in the presented energy values. The calculations were performed using the Gaussian 16 software [35].
Molecular ions containing up to 7 Au atoms were studied and, depending on the number of Au atoms, up to 11 H atoms. As the system size increases, so too does the number and complexity of stable isomers as well as the computational resources required. This was the main limitation to the sizes of systems that we have studied. We have investigated a wide range of possible structures for our mixed clusters, including the structures for pure gold clusters from Schooss et al. [27] as initial guesses for the optimizations, and for certain cluster sizes, the preferred structure of the gold atoms changed depending on the number of hydrogens added. A comparison of the pure gold cluster structures and the structures for the magic numbers can be found in the SI.
Results and Discussion
Observation of Hydrogenated Gold Cluster Cations
Figure 2 shows the intensity distribution obtained mass spectrometrically for the hydrogenated gold cluster cations AunHx+ seen with n up to 15. A clear oscillation in intensity is seen, with odd-numbered clusters generally being more intense than even numbered clusters. With Aun+ carrying the positive charge, we note that the odd-numbered clusters are even electron systems while the even-numbered clusters are odd electron systems. No doubly charged ions were visible, nor has their observation ever been reported before by others, as far as we are aware.
Positive ion mass spectrum obtained by electron ionization of helium nanodroplets showing the pure and mixed gold cluster AunHx+ peaks. The coarse structure at the lower mass range is composed mainly by pure He clusters. He droplets with a size of about 106 atoms were generated with a nozzle temperature of 9.55 K and a stagnation pressure of 2.25 MPa. They were doped first with H2 molecules and then with Au atoms. Pickup pressure was 1.18 × 10−3 Pa for H2. The metal oven was heated with 118 W. The electron energy for the ionization process was 85 eV. The inset is a close-up on part of the Au3+ cluster series where the added hydrogens can easily be distinguished. We also see some residual water molecules binding to the gold clusters
Ion Yields
Au doping of HNDs leads to the formation of pure clusters of Au atoms, Aun, and when hydrogen is present as well, the formation of hydrogenated clusters Aun(H2)m. The strongly bonded H2 molecules are not expected to dissociate in the presence of the gold clusters at the low temperature of the HNDs; the reaction of molecular hydrogen with gold atoms to produce AuH is known to be endothermic by more than 1 eV [19].
When the HNDs are exposed to electron impact ionization and He+ ions are formed, both Aun and Aun(H2)m clusters can become ionized by electron transfer to these He+ ions (IE[He] = 24.59 eV [36]). We have previously reported the formation of positive clusters of gold atoms under similar conditions in the absence of molecular hydrogen [37]. The excess energy of the electron transfer heats up the charged clusters and can promote its fragmentation. Some cluster ions with a longer lifetime will demonstrate enhanced stability as a consequence of more efficient quenching by the ultra-cold helium matrix. Other precursors of cluster ionization include metastable helium atoms He* as well as He*− or by proton transfer from HenHx+ or Hx+ derived from He+ reactions with H2 [22] leading to AunH(H2)m+ cations.
Figure 3 presents the influence of the energy of the electrons impacting the HNDs, doped with Au and H2, on the relative ion yield of protonated cluster ions AunH+ with n = 3, 6, 9, and 12 and of hydrogenated gold cluster ions AunH4+ with n = 3, 6, 9, and 12. Both populations exhibit an onset around 20 eV, near the 19.8 eV required to form He* in its lowest lying excited state, and there seem to be no remarkable differences in the shapes of the ion profiles. Interestingly, this differs somewhat from the behavior of the ion efficiency curves for pure gold cluster cations, where the position of the maxima shifts towards lower electron energies with increasing cluster size [29]. The reason for this difference is not entirely clear, but could be because of a difference in mean droplet size or by the presence of H2 in the droplets.
Ion efficiency curves for selected protonated gold clusters, AunH+ (left panel), and selected hydrogenated gold cluster cations AunHx+ (right panel) with x = 4 formed upon electron impact on HNDs doped with hydrogen and gold. Helium temperature before expansion was 9.55 K and the pressure 2.25 MPa. The hydrogen pressure was 1.11 × 10−3 Pa and the power of the gold oven was 118 W
Computed Ionization Energies and Proton Affinities of Gold Clusters
Because of the excess energy available in both the electron and proton transfer reactions, AunH(H2)m+ formation can be accompanied by the dissociation of the cluster ions through H2 elimination with, as we shall see, the ultimate preferred formation of “magic” hydrogenated clusters of special stability. The excess energy in the ionization of Aun(H2)m can be considerable because of the high recombination energy of, for example, He+ (24.59 eV). Similarly, the very low proton binding energy of the proton donors, e.g., HeH+ or H3+ with PA(He) = 1.82 eV [38] and PA(H2) = 4.39 eV [39], leads to high excess energies in the proton transfer to the gold clusters in secondary HeH+/H3+ + Aun → HeH/H3 + AunH+ reactions that may drive the annealing of the final mixed cluster products.
Ionization energies of small pure gold clusters and their variation with size have been reported previously in the literature, but little appears to be known about the proton affinities and their variation with size. The variation of ionization energy of Aun for n = 1–22, known in 2003, has been graphed by Sugawara et al [21]. A striking even/odd oscillation with cluster size n, with even-n clusters relatively more predominant, is clearly evident and the authors remark on how this oscillation matches that observed in the binding energy De of Aun+-Au. The results of our calculations of IE are summarized in Table 1 and plotted in Figure 1. There is agreement as regards both magnitudes and even/odd oscillations in IE. Also included in Table 1 and Figure 1 are our computed values for the proton affinities of the gold clusters. Of note is the sharp increase in PA for clusters with n > 1.
Observed Profiles of Hydrogenation for Individual Gold Cluster Sizes
Figure 5 provides panels that show the distribution in hydrogenation observed in our experiments for AunHx+ cluster sizes from n = 1 to 8 and x from 1 up to 20. These distributions exhibit oscillations and the presence of intense “magic” numbers that shift to higher hydrogenation for clusters with up to 5 gold atoms. Oscillations appear to be more pronounced for odd-numbered gold clusters and at lower degrees of hydrogenation. They are still present for clusters with 6 to 8 gold atoms but strong magic numbers are less pronounced in relative intensity. Another striking feature is the shift from the very pronounced magic numbers that can be seen for n ≤ 5 to the richer intensity distributions for n ≥ 6. For example, there is a sharp drop in ion yield after Au6H9+, but also rather high intensities of ions with fewer H atoms. This could be an indication of a transition from 2D to 3D structures as the cluster sizes increase, leading to more possible isomers being available, each contributing with their own different magic combinations of Au and H atoms.
We note the following hydrogenation features for specific gold cluster ions:
n = 1: Odd-numbered AuHx+ are more intense than their even-numbered neighbors with the notable exception of AuH4+ which clearly exhibits special stability.
n = 2: Au2H5+ clearly predominates and Au2H6+ also has a relatively high intensity compared to all other less remarkable Au2Hx+ cluster sizes.
n = 3: The early even-numbered Au3Hx+ ions are observed to increase in intensity from x = 0 to 2 to 4 to 6. Also for the odd-numbered Au3Hx+ ions, an increase can be observed from x = 1 to 3 to 5 to 7 with odd x Au3Hx+ ions being less intense than the preceding even x ions. The Au3H6+ ion is the most intense overall.
n = 4: This time, the early Au4Hx+ are observed to increase in intensity from x = 0 to 7, with a local minimum at x = 2 and even numbered ions being less intense than preceding odd x ions. The Au4H7+ ion is the most intense overall. Au4Hx+ cluster sizes with x = 9 to 20 are of low intensity but exhibit a clear odd-even oscillation, with minima at even numbers of H atoms x.
n = 5: As for n = 3, the early even-numbered Au5Hx+ ions are observed to increase in intensity from x = 2 to 4 to 6 to 8 and odd x Au5Hx+ ions being less intense than neighboring even x ions. Au5H8+ and Au5H7+ are the most intense even and odd x ions, respectively, with the former being the most intense overall.
n = 6: After the initial appearance of the protonated cluster Au6H+, oscillations are seen with local maxima of the ion yield of Au6Hx+ at x = 5 and 8, with Au6H8+ being slightly more predominant. A sharp drop-off in ion intensity is seen after Au6H9+. Curiously, a “rogue” cluster ion Au6H17+ shows a small maximum beyond Au6H11+.
n = 7: Four strong oscillations are seen early on for the even x cluster ions Au7Hx+ with x = 2, 4, 6, and 8 with x = 6 predominating. The ion yields for cluster ions Au7Hx+ with x > 9 exhibit no odd-even oscillation.
n = 8: A strong protonated cluster peak Au8H+ is followed by strong adduct peaks with one and two H2 molecules. Note from Table 1 and Figure 4 that the calculations indicate that Au8 has the highest proton affinity (7.76 eV) of the systems studied here.
Figure 5 also includes the FT-ICR data of Sugawara et al. [21] obtained in experiments with the laser ablation of gold in a H2(5.3%)/He mixture (gray bars). Hydride gold cluster distributions are observed that are sometimes similar but more often distinctly different from ours. The extent of hydrogenation is generally seen to be much smaller, but the presence of magic number intensities for Au2H5+ and Au3H6+ coincides with ours. Magic numbers in the FT-ICR spectra are also observed otherwise, but generally shifted to lower hydrogenation. These differences may well be due to the higher temperature of the FT-ICR experiments, direct formation of Aun+ cluster ions by laser ablation, and a significant presence of H atoms in the Aun+ cluster ion formation region.
Cluster series of AunHx+ from n = 1 to 8 and x from 1 up to 20, extracted from the mass spectrum shown in Figure 1 using the IsotopeFit software [34]. Magic number peaks are clearly visible as are odd-even oscillations corresponding to clusters with H2 molecules in the presence or absence of an H atom. The bar peaks represent the FT-ICR data of Sugawara et al. [21]
Computed Structures of Hydrogenated Gold Cluster Cations
Figure 6 displays the structures for some of the most abundant “magic” clusters of AunHx+ for each series of n = 1 to 7 calculated at the MP2/def2-TZVP level of theory. The even-numbered gold cluster cations are open shell radicals and thus preferentially protonated. In general, the protonated gold clusters (AunH+) have structures that are very similar to the next larger (closed shell) pure gold cluster cations (Aun+1+). All the structures that are shown are planar, in regard to the positions of the Au atoms, except Au7H6+, which agrees with the nonplanar Au7+ structure. Comparisons between the structures in Figure 6 and the bare Aun+ structures can be found in the SI.
For each combination of Au and H, several structures were optimized to find the one with the lowest potential energy. In the cases of the “magical” structures, alternative isomers found have at least 0.1 eV higher energy than the proposed minima.
The calculations suggest that H2 molecules bond directly to Au atoms of the gold cluster “skeleton” and that the extra H atom in the even-numbered gold clusters (n = 2, 4, and 6) simply bridges two Au atoms.
Computed Energies of Hydrogenated Gold Cluster Cations
The odd-even oscillations seen in the data shown in Figure 5 correspond to cluster cations with added intact H2 molecules in the presence or absence of an H atom. In our calculations, we explored the H2 affinities (∆E0) of the gold cluster cations for molecular hydrogen. The results are summarized in Table 2 and graphed in Figure 7. Hydrogenation with H2 molecules was seen to be limited with larger cluster cations exhibiting a greater capacity for hydrogenation but weaker bonding of individual hydrogen molecules. Up to two H2 molecules bind strongly to Au+ and Au2H+ with energies of 0.8 to 1.1 eV. Au3+ and Au4H+ have a significant affinity for up to three molecules of H2, the first two with about 0.7 eV and the third somewhat lower still by 0.2 and 0.3 eV, respectively. The H2 affinities of Au5+, Au6H+, and Au7+ are the lowest, below 0.68 eV, but the trends suggest that the “magic” numbers seen in the experiments correspond to gold clusters that are saturated with a first layer of relatively strongly bound H2 units.
Conclusions
Our experiments have shown that H2 molecules readily attach to gold clusters with up to at least 8 gold atoms in a He environment near zero K. These hydrogenated clusters are readily ionized in the presence of electron acceptors such as He+ or proton donors such as HeH+ and some H2 elimination may ensue due to the high excess energy of these processes. There was no evidence for the dissociation of adsorbed H2 molecules; there was no indication of H elimination that might result from dissociation. The hydrogenated gold cluster ion distributions exhibit “magic” features that appear to reflect special stabilities for certain numbers of H2 adsorbed molecules.
Our calculations have indicated that the number, including the “magic” number of H2 adsorbed molecules, is determined by the structure of the underlying (most often flat) Au cluster skeleton and the number of Au atoms exposed on the periphery. The computed H2 affinities of the cation clusters are as high as 1.1 eV, but weaken with increasing cluster size. H atoms appear to bridge two Au atoms in hydrogenated clusters with an even number of Au atoms.
References
Claus, P.: Heterogeneously catalysed hydrogenation using gold catalysts. Appl. Catal. A Gen. 291, 222–229 (2005)
Fujitani, T., Nakamura, I., Akita, T., Okumura, M., Haruta, M.: Hydrogen dissociation by gold clusters. Angew. Chem. Int. Edit. 48, 9515–9518 (2009)
Haruta, A.: When gold is not noble: catalysis by nanoparticles. Chem. Rec. 3, 75–87 (2003)
Sanchez, A., Abbet, S., Heiz, U., Schneider, W.D., Häkkinen, H., Barnett, R.N., Landman, U.: When gold is not noble: nanoscale gold catalysts. J. Phys. Chem. A. 103, 9573–9578 (1999)
Zhao, S., Jin, R.X., Abroshan, H., Zeng, C.J., Zhang, H., House, S.D., Gottlieb, E., Kim, H.J., Yang, J.C., Jin, R.C.: Gold nanoclusters promote electrocatalytic water oxidation at the nanocluster/CoSe2 interface. J. Am. Chem. Soc. 139, 1077–1080 (2017)
Haruta, M.: Size- and support-dependency in the catalysis of gold. Catal. Today. 36, 153–166 (1997)
Tyo, E.C., Vajda, S.: Catalysis by clusters with precise numbers of atoms. Nat. Nanotechnol. 10, 577–588 (2015)
Schlangen, M., Schwarz, H.: Effects of ligands, cluster size, and charge state in gas-phase catalysis: a happy marriage of experimental and computational studies. Catal. Lett. 142, 1265–1278 (2012)
Wang, L.-S.: Covalent gold. Phys. Chem. Chem. Phys. 12, 8694–8705 (2010)
Zavras, A., Khairallah, G.N., O’Hair, R.A.J.: Gas phase formation, structure and reactivity of gold cluster ions. In: Mingos D.M.P. (ed.) Gold clusters, colloids and nanoparticles II, pp. 139, Springer International Publishing, (2014)
Woodham, A.P., Fielicke, A.: Gold clusters in the gas phase. In: Mingos D.M.P. (ed.) Gold clusters, colloids and nanoparticles I, pp. 243. Springer International Publishing, (2014)
Jordan, A.J., Lalic, G., Sadighi, J.P.: Coinage metal hydrides: synthesis, characterization, and reactivity. Chem. Rev. 116, 8318–8372 (2016)
Lang, S.M., Bernhardt, T.M., Chernyy, V., Bakker, J.M., Barnett, R.N., Landman, U.: Selective C−H bond cleavage in methane by small gold clusters. Angew. Chem. Int. Ed. 56, 13406–13410 (2017)
Mostowitsch, W., Pletneff, W.: Volatility of gold at high temperatures in atmospheres of air and other gases. J. Russ. Metall. Soc. 3, 410–431 (1915)
Hulthèn, E., Zumstein, R.V.: The absorption spectra of some hydride compounds in the ultra-violet. Phys. Rev. 28, 13–24 (1926)
Khairallah, G.N., O'Hair, R.A.J., Bruce, M.I.: Gas-phase synthesis and reactivity of binuclear gold hydride cations, (R3PAu)2H+ (R = Me and Ph). Dalton Trans. 0, 3699–3707 (2006)
Schmidbaur, H., Raubenheimer, H.G., Dobrzańska, L.: The gold–hydrogen bond, Au–H, and the hydrogen bond to gold, Au⋯H–X. Chem. Soc. Rev. 43, 345–380 (2014)
Varganov, S.A., Olson, R.M., Gordon, M.S., Mills, G., Metiu, H.: A study of the reactions of molecular hydrogen with small gold clusters. J. Chem. Phys. 120, 5169–5175 (2004)
Wang, X.F., Andrews, L.: Gold hydrides AuH and (H2)AuH and the AuH3 transition state stabilized in (H2)AuH3: infrared spectra and DFT calculations. J. Am. Chem. Soc. 123, 12899–12900 (2001)
Andrews, L., Wang, X.F., Manceron, L., Balasubramanian, K.: The gold dihydride molecule, AuH2: calculations of structure, stability, and frequencies, and the infrared spectrum in solid hydrogen. J. Phys. Chem. A. 108, 2936–2940 (2004)
Sugawara, K., Sobott, F., Vakhtin, A.B.: Reactions of gold cluster cations Aun + (n=1-12) with H2S and H2. J. Chem. Phys. 118, 7808–7816 (2003)
Mauracher, A., Echt, O., Ellis, A.M., Yang, S., Bohme, D.K., Postler, J., Kaiser, A., Denifl, S., Scheier, P.: Cold physics and chemistry: collisions, ionization and reactions inside helium nanodroplets close to zero K. Phys. Rep. 751, 1–90 (2018)
Haberfehlner, G., Thaler, P., Knez, D., Volk, A., Hofer, F., Ernst, W.E., Kothleitner, G.: Formation of bimetallic clusters in superfluid helium nanodroplets analysed by atomic resolution electron tomography. Nat. Commun. 6, 8779 (2015)
Messner, R., Schiffmann, A., Pototschnig, J.V., Lasserus, M., Schnedlitz, M., Lackner, F., Ernst, W.E.: Spectroscopy of gold atoms and gold oligomers in helium nanodroplets. J. Chem. Phys. 149, 13 (2018)
Volk, A., Thaler, P., Knez, D., Hauser, A.W., Steurer, J., Grogger, W., Hofer, F., Ernst, W.E.: The impact of doping rates on the morphologies of silver and gold nanowires grown in helium nanodroplets. Phys. Chem. Chem. Phys. 18, 1451–1459 (2016)
Feng, C., Latimer, E., Spence, D., Al Hindawi, A., Bullen, S., Boatwright, A., Ellis, A.M., Yang, S.F.: Formation of Au and tetrapyridyl porphyrin complexes in superfluid helium. Phys. Chem. Chem. Phys. 17, 16699–16704 (2015)
Schöbel, H., Bartl, P., Leidlmair, C., Denifl, S., Echt, O., Märk, T.D., Scheier, P.: High-resolution mass spectrometric study of pure helium droplets, and droplets doped with krypton. Eur. Phys. J. D. 63, 209–214 (2011)
Goulart, M., Gatchell, M., Kranabetter, L., Kuhn, M., Martini, P., Gitzl, N., Rainer, M., Postler, J., Scheier, P., Ellis, A.M.: The adsorption of helium atoms on small cationic gold clusters. Phys. Chem. Chem. Phys. 20, 9554–9560 (2018)
Goulart, M., Kuhn, M., Martini, P., Chen, L., Hagelberg, F., Kaiser, A., Scheier, P., Ellis, A.M.: Highly stable C60AuC60 +/− dumbbells. J. Phys. Chem. Lett. 9, 2703–2706 (2018)
Sliter, R., Gomez, L.F., Kwok, J., Vilesov, A.: Sizes distributions of large He droplets. Chem. Phys. Lett. 600, 29–33 (2014)
Toennies, J.P., Vilesov, A.F.: Superfluid helium droplets: a uniquely cold nanomatrix for molecules and molecular complexes. Angew. Chem. Int. Ed. 43, 2622–2648 (2004)
Mauracher, A., Daxner, M., Huber, S.E., Postler, J., Renzler, M., Denifl, S., Scheier, P., Ellis, A.M.: The interaction of He− with fullerenes. J. Chem. Phys. 142, 104306 (2015)
Mauracher, A., Daxner, M., Postler, J., Huber, S.E., Denifl, S., Scheier, P., Toennies, J.P.: Detection of negative charge carriers in superfluid helium droplets: the metastable anions He*− and He2*. J. Phys. Chem. Lett. 5, 2444–2449 (2014)
Ralser, S., Postler, J., Harnisch, M., Ellis, A.M., Scheier, P.: Extracting cluster distributions from mass spectra: IsotopeFit. Int. J. Mass Spectrom. 379, 194–199 (2015)
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Petersson, G.A., Nakatsuji, H., Li, X., Caricato, M., Marenich, A.V., Bloino, J., Janesko, B.G., Gomperts, R., Mennucci, B., Hratchian, H.P., Ortiz, J.V., Izmaylov, A.F., Sonnenberg, J.L., Williams D, Ding, F., Lipparini, F., Egidi, F., Goings, J., Peng, B., Petrone, A., Henderson, T., Ranasinghe, D., Zakrzewski, V.G., Gao, J., Rega, N., Zheng, G., Liang, W., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Throssell, K., Montgomery Jr., J.A., Peralta, J.E., Ogliaro, F., Bearpark, M.J., Heyd, J.J., Brothers, E.N., Kudin, K.N., Staroverov, V.N., Keith, T.A., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A.P., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Millam, J.M., Klene, M., Adamo, C., Cammi, R., Ochterski, J.W., Martin, R.L., Morokuma, K., Farkas, O., Foresman, J.B., Fox, D.J.: Gaussian 16 Revison A.03. (2016), Gaussian Inc. Wallingford, CT
Martin, W.C.: Improved 4He I 1snl ionization-energy, energy-levels, and lamb shifts for 1sns and 1snp terms. Phys. Rev. A. 36, 3575–3589 (1987)
Martini, P., Kranabetter, L., Goulart, M., Kuhn, M., Gatchell, M., Bohme, D.K., Scheier, P.: Formation of positive and negative clusters of gold atoms inside helium nanodroplets close to zero K. Int. J. Mass Spectrom. 434, 136–141 (2018)
Beauchamp, J.L. In: Ausloos, P. (ed.) Interaction between Ions and Molecules, pp. 413. Springer US. Plenum Press, New York (1975)
Burt, J.A., Dunn, J.L., McEwan, M.J., Sutton, M.M., Roche, A.E., Schiff, H.I.: Some ion-molecule reactions of H3 + and proton affinity of H2. J. Chem. Phys. 52, 6062–6075 (1970)
Acknowledgements
Open access funding provided by Austrian Science Fund (FWF). This work was supported by the Austrian Science Fund, FWF (project numbers P23657, P30355, P31149), the European Commission (ELEvaTE H2020 Twinning Project, project number 692335), and the Swedish Research Council (contract No. 2016-06625).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Helmut Schwarz on his election to the National Academy of Science.
Electronic supplementary material
ESM 1
(DOCX 816 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lundberg, L., Martini, P., Goulart, M. et al. Hydrogenated Gold Clusters from Helium Nanodroplets: Cluster Ionization and Affinities for Protons and Hydrogen Molecules. J. Am. Soc. Mass Spectrom. 30, 1906–1913 (2019). https://doi.org/10.1007/s13361-019-02235-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-019-02235-1