Abstract
Proton transfer reaction mass spectrometry (PTR-MS) has played an important role in the field of real-time monitoring of trace volatile organic compounds (VOCs) due to its advantages such as low limit of detection (LOD) and fast time response. Recently, a new technology of proton extraction reaction mass spectrometry (PER-MS) with negative ions OH– as the reagent ions has also been presented, which can be applied to the detection of VOCs and even inorganic compounds. In this work, we combined the functions of PTR-MS and PER-MS in one instrument, thereby developing a novel technology called dipolar proton transfer reaction mass spectrometry (DP-PTR-MS). The selection of PTR-MS mode and PER-MS mode was achieved in DP-PTR-MS using only water vapor in the ion source and switching the polarity. In this experiment, ketones (denoted by M) were selected as analytes. The ketone (molecular weight denoted by m) was ionized as protonated ketone [M + H]+ [mass-to-charge ratio (m/z) m + 1] in PTR-MS mode and deprotonated ketone [M – H]– (m/z m – 1) in PER-MS mode. By comparing the m/z value of the product ions in the two modes, the molecular weight of the ketone can be positively identified as m. Results showed that whether it is a single ketone sample or a mixed sample of eight kinds of ketones, the molecular weights can be detected with DP-PTR-MS. The newly developed DP-PTR-MS not only maintains the original advantages of PTR-MS and PER-MS in sensitive and rapid detection of ketones, but also can estimate molecular weight of ketones.

ᅟ
Similar content being viewed by others
Introduction
Proton transfer reaction mass spectrometry (PTR-MS) is a chemical ionization (CI) mass spectrometry that can be used for on-line detection of trace volatile organic compounds (VOCs). Because it has the advantages of short response time, low limit of detection (LOD), direct sampling, etc. [1–5], PTR-MS is widely used in many fields, such as environmental protection, food, medicine, and public safety [6–13].
In traditional PTR-MS, H3O+ ions generated from water vapor with a discharge source are used as proton donor ions. The VOCs are ionized by undergoing proton transfer reaction with the H3O+. Although this is a soft ionization technique, it is inevitable that a few cluster ions and fragmental ions are produced, which causes difficulty in qualitatively identifying the VOCs. Therefore, successors exploited O2 +, NO+, and NH4 + as reagent ions, and the identification of VOCs was improved, owing to the production of different product ions resulting from changes in the reagent ion-molecule chemistry [14–19]. However, these reagent ions must be prepared with specific reagent gasses (oxygen, dry air, ammonia).
Based on previous studies [20, 21], our research team recently developed a new proton extraction reaction mass spectrometry (PER-MS) that uses OH– ions as reagent ions, prepared with only water vapor in the discharge source [22]. The detection of VOCs with PER-MS is based on the proton extraction reaction. With the development of this technology, it is possible to prepare different reagent ions using only water vapor.
Based on our previous research on PTR-MS and PER-MS [14, 15, 22], a new technology called dipolar proton transfer reaction mass spectrometry (DP-PTR-MS) is presented in this work. DP-PTR-MS prepares two kinds of reagent ions (H3O+ and OH-) with only water vapor discharged in a hollow cathode (HC) ion source, and the selection of PTR-MS mode and PER-MS mode was achieved by a circuit switch in the single instrument. The water vapor flow and reduced field in the drift tube were optimized. Ketone was tested as an example, and a single kind sample and mixed samples of eight kinds of ketones (acetone, 2-butanone, 2-pentanone, 2-hexanone, 2-heptanone, 2-octanone, cyclopentanone, and cyclohexanone) were analyzed, respectively, in the two modes of DP-PTR-MS. With the comparison of the characteristic ions for one ketone in two modes, the molecular weight of this ketone can be estimated with DP-PTR-MS.
Experimental
Instrumentation and Principle
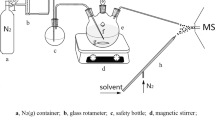
DP-PTR-MS mainly consists of an ion source, drift tube, intermediate chamber, and ion detection system. The schematic view of the DP-PTR-MS instrument is shown in Figure 1. The power supply equipment for the ion source, drift tube, and ion lens in the intermediate chamber is a self-developed multichannel DC power supply. The output voltage of every channel can be individually adjusted, and the positive and negative polarities of each channel also can be reversed. The ion detection system has positive and negative ion detection modes, which can be switched with the software. The selection of PTR-MS mode and PER-MS mode can be achieved by changing the polarity of multi channel DC power supply and the mode of the ion detection system.
PTR-MS Mode
In PTR-MS mode, high concentrations of H3O+ ions and OH– ions are generated from water vapor discharged in the HC ion source. The H3O+ ions are introduced into the drift tube with the positive electric field, and can undergo proton transfer reaction with the ketone (denoted by M), which comes from the upstream part of the drift tube, as long as the ketone’s proton affinity (PA) exceeds that of H2O(PAH2O = 691 kJ/mol)[23]. The protonated M can be detected in the positive ion detection mode of the ion detection system.
PER-MS Mode
In PER-MS mode, high concentrations of H3O+ and OH– ions are produced from water vapor discharged in the same HC ion source. The OH– ions are introduced into the drift tube with a negative electric field and undergo a proton extraction reaction with the M coming from the upstream of the drift tube. The deprotonated M can be detected in the negative ion detection mode of the ion detection system.
In this experiment, the pressure in the drift tube was 1.75 mbar in PTR-MS mode and 1.95 mbar in PER-MS mode. The temperature of drift tube was kept at 338 K. The pressure in the ion detection system was about 6.3 × 10–7 mbar. The voltage of the drift tube could be adjusted from 0 to 1000 V.
The sample was introduced into a 20 cm capillary through the bypass of a tee fitting and then introduced into the drift tube. The sample flow in the capillary was 2.84 mLmin–1. The temperature of the capillary was kept at 373 K.
Reagents and Samples
Acetone used in this experiment was purchased from Shanghai Zhenqi Chemical Reagent Co., Ltd., China; 2-butanone, 2-pentanone, 2-hexanone, 2-heptanone, and 2-octanone were purchased from Shanghai Jingchun Reagent Co., Ltd. Cyclopentanone and cyclohexanone were purchased from National Pharmaceutical Group Shanghai Chemical Reagent Co., Ltd., China. All these reagents are analytically pure. The high-purity water used to generate water vapor was produced by combining two lab water purification systems (KNTR-I-10 and Micropure UF). High-purity nitrogen (99.999%) was purchased from Nanjing Special Gas Factory Co., Ltd., China. The syringe pump (model no. 601553) was purchased from KD Scientific, Holliston, USA.
The gas samples of ketones detected with DP-PTR-MS were obtained by diluting the saturated vapor of ketones with high-purity nitrogen gas. The flow of high-purity nitrogen was controlled by a flowmeter with a valve. The saturated vapor of ketone was pushed into the high-purity nitrogen by a syringe pump. The concentration of ketone could be prepared to an appropriate value by adjusting the push velocity of the syringe pump and the flow of the high-purity nitrogen. This method can ensure that the product ions’ intensity I PI was far less than the reagent ions’ intensity I RI (I PI /I RI was less than 10%).
Results and Discussion
Parameter Optimization
Optimization of the Water Vapor Flow
The water vapor flow through the ion source has a great effect on the ion intensity of the reagent ions, so the ion intensity of reagent ions was monitored at different water vapor flow rates to optimize this parameter. To protect the secondary electron multiplier (SEM), the H3 18O+ and 18OH- reagent ions were monitored instead of the H3 16O+ and 16OH- reagent ions, respectively. High-purity nitrogen was used as the carrier gas to exclude interference of compounds in the air. The reduced field E/N in the drift tube was kept at 76.8 Td (where E is the electric field across the drift tube and N is the gas number density in the drift tube; 1 Td = 10–17 V cm2). The optimization results of water vapor flow are shown in Figure 2. In PTR-MS mode, when the water vapor flow is 0.7 mL/min–1, the intensity of the ion at m/z 21 (H3 18O+) reaches its maximum, as shown in Figure 2a. At PER-MS mode, the intensity of the ion at m/z 19 (18OH–) is highest when the water vapor flow is 2.8 mL min–1, as shown in Figure 2b.
(a) Dependence of the intensity of the ion at m/z 21 (H3 18O+) at PTR-MS mode on the water vapor flow. The water vapor flow was set at 0.175, 0.35, 0.7, 1.05, 1.4, 1.75 mL min–1. (b) Dependence of the intensity of the ion at m/z 19 (18OH–) at PER-MS mode on the water vapor flow. The water vapor flow was set at 1.4, 1.75, 2.1, 2.45, 2.8, 3.15 mL min–1
Optimization of Reduced Field
In PTR-MS, the reduced field E/N in the drift tube is usually set to 100–120 Td to minimize the fragmental ions [24]. However, some protonated VOCs are difficult to be observed using this conventional reduced field, which means that the reduced field should be optimized for each particular compound. Accordingly, 2-pentanone was used to optimize the reduced field for detecting ketones. Figure 3a shows the relative abundances of seven kinds of product ions at different reduced fields E/N in PTR-MS mode. With the decrease of E/N, the relative abundances of protonated 2-pentanone (m/z 87) begin to increase noticeably at E/N = 80 Td. The relative abundance of protonated 2-pentanone (m/z 87) ions reaches its maximum at about E/N = 60 Td. Moreover, the relative abundances of some fragmental ions increase with the decrease of the reduced field E/N. When the E/N is as low as 53.1 Td, the fragmental ions still exist. Reducing the reduced field E/N does not make the fragmental ions of 2-pentanone disappear. Figure 3b shows the change of relative distributions of the four kinds of product ion (m/z 57, 59, 71, 85) of 2-pentanon at different reduced fields E/N in PER-MS mode. When the reduced field E/N value is less than 78.3 Td, the relative abundance of the protonated 2-pentanone at m/z 87 is more than 90% and the relative abundance of other product ions at m/z 57, 59, and 71 are lower than 5%. Therefore, the reduced field E/N was set at 78.3 Td in the following experiments.
The Background of Mass Spectrum in DP-PTR-MS
The water vapor flow was set at the optimized value in the following experiment. High-purity nitrogen was used as the carrier gas in the sample inlet and the drift tube in DP-PTR-MS. The mass spectrum of full scans from m/z 20 to 140 at E/N = 78.3 Td is shown in Figure 4. In PTR-MS mode, the main mass peak in the background of the mass spectrum is attributed to the proton donor H3O+ ions, which can be estimated by the intensity of H3 18O at m/z 21. However, there is still a small amount of NO+ at m/z 30 in the drift tube, and another obvious peak at m/z 37 should be attributed to water cluster ions (H2O) H3O+ as shown in Figure 4a [25, 26]. In PER-MS mode, most of the ions extracted by the negative electric field are proton acceptor OH– ions but several peaks at m/z 35 (possibly attributable to (H2O)OH– or 35Cl–), m/z 37 (possibly attributable to 37Cl–), m/z 46 (possibly attributable to NO2 –), and m/z 61 (possibly attributable to (CO2)OH–) can also be clearly seen in the mass spectrum as shown in Figure 4b [22]. However, the small amount of these impurity ions leads to only negligible interference for the detection of ketones in this experiment.
The Detection of Ketones
In this research, we used ketones as examples. Acetone, 2-butanone, 2-pentanone, 2-hexanone, 2-heptanone, 2-octanone, cyclopentanone, and cyclohexanone were detected by the optimized DP-PTR-MS. The detection results with high-purity nitrogen as the carrier gas are shown in Figure 5. It can be seen that the product ions with the maximal m/z values in the two modes are protonated ketone (m/z m + 1) and deprotonated ketone (m/z m – 1). For example, the characteristic ions of acetone are positive ions at m/z 59 and negative ions at m/z 57 (Figure 5a). The characteristic ions of 2-butanone are positive ions at m/z 73 and negative ions at m/z 71 (Figure 5b). The characteristic ions of 2-pentanone are positive ions at m/z 87 and negative ions at m/z 85 (Figure 5c). The characteristic ions of 2-hexanone are positive ions at m/z 101 and negative ions at m/z 99 (Figure 5d). The characteristic ions of 2-heptanone are positive ions at m/z 115 and negative ions at m/z 113 (Figure 5e). The characteristic ions of 2-octanone are positive ions at m/z 129 and negative ions at m/z 127 (Figure 5f). The characteristic ions of cyclopentanone are positive ions at m/z 85 and negative ions at m/z 83 (Figure 5g). The characteristic ions of cyclohexanone are positive ions at m/z 99 and negative ions at m/z 97 (Figure 5h). By comparing the product ions with the maximum m/z in the two modes, the molecular weight of this ketone can be identified as m.
The mass spectrum of (a) acetone, (b) 2-butanone, (c) 2-pentanone, (d) 2-hexanone, (e) 2-heptanone, (f) 2-octanone, (g) cyclopentanone, (h) cyclohexanone detected by DP-PTR-MS. The black-labeled peaks are the background spectrum peaks. The blue-labeled peaks are the spectrum peaks of protonated ketones and the spectrum peaks of deprotonated ketones. The red-labeled peaks are the mass spectrum of the fragmental ions
Although the peaks of the quasi-molecular ions (at m/z m + 1, m – 1) can be observed in both modes, obvious fragmental ions of these ketones (except for acetone) were also observed in PTR-MS mode. There were even eight kinds of fragmental ions for 2-heptanone. For 2-pentanone, 2-hexanone, 2-heptanone, 2-octanone, and cyclohexanone in PTR-MS mode, the highest intensity peak in the mass spectrum is not the quasi-molecular ion peak. However, in PER-MS mode, the relative abundance of quasi-molecular ions at m/z m – 1 is more than 90%, and the intensities of their fragmental ions are negligible. This shows that DP-PTR-MS can detect ketones in both modes and can help to identify their molecular weight. Additionally, the new PER-MS mode has more effective soft ionization ability for ketones.
DP-PTR-MS can detect ketones not only in samples with a single ketone, but also in mixed ketone samples. A mixed solution was prepared with the above eight kinds of ketones and left standing for half an hour, then the headspace gas of the mixed solution was extracted as the mixed sample. Using the same experimental conditions above, the mixed sample was analyzed by DP-PTR-MS; the results are shown in Figure 6. The ionic peaks at m/z 59, 73, 85, 87, 99, 101, 115, and 129 in the mass spectrum obtained in PTR-MS mode belong to protonated acetone, 2-butanone, cyclopentanone, 2-pentanone, cyclohexanone, 2-hexanone, 2-heptanone, and 2-octanone, respectively. The other ions detected in PTR-MS mode are fragmental ions. The corresponding quasi-molecular ion peaks in PER-MS mode are the ionic peaks at m/z 57, 71, 83, 85, 97, 99, 113, and 127, which are the eight kinds of deprotonated ketones. This also shows that DP-PTR-MS offers a better choice for the detection of mixed sample of ketones in PER-MS mode even when there is possible interference from fragmental ions in PTR-MS mode.
Conclusion
This work introduces a novel DP-PTR-MS technology that combines the functions of PTR-MS and PER-MS. The switching between the two modes is simple, without any complex tasks such as preparing multiple reactive gasses. Since both reagent ions, H3O+ and OH–, were generated by water vapor discharge in a single ion source, there is no pollution or interference from different reagent gasses caused by switching between two modes. The same substance underwent different ion molecular reactions (proton transfer reaction and proton extraction reaction) in the two modes to produce different characteristic ions. Taking ketones as examples, the molecular weight of ketones, even in a mixture, can be estimated by comparing the m/z values of the two kinds of characteristic ions in the two modes. In this work, the conditions of water vapor flow and reduced field were optimized. In PTR-MS mode and PER-MS mode, the optimal water vapor flow was 0.7 mL min–1 and 2.8 mL min–1, respectively, and the optimal reduced field E/N was 78.3 Td. DP-PTR-MS could detect molecular weight in single-ketone samples and in mixed samples containing eight kinds of ketones. The PER-MS mode in DP-PTR-MS has more obvious advantages for the detection of mixed sample of ketones. The novel DP-PTR-MS may have important application value in the field of VOC detection.
References
Jin, S.P., Li, J.Q., Han, H.Y., Wang, H.M., Chu, Y.N., Zhou, S.K.: Proton transfer reaction mass spectrometry for online detection of trace volatile organic compounds. Prog. Chem. 19, 996–1006 (2007)
Romano, A., Capozzi, V., Spano, G., Biasioli, F.: Proton transfer reaction-mass spectrometry: online and rapid determination of volatile organic compounds of microbial origin. Appl. Biochem. Biotechnol. 99, 3787–3795 (2015)
Lindinger, W., Hansel, A., Jordan, A.: Proton-transfer-reaction mass spectrometry (PTR-MS): on-line monitoring of volatile organic compounds at pptv levels. Chem. Soc. Rev. 27, 347–354 (1998)
Graus, M., Mueller, M., Hansel, A.: High resolution PTR-TOF: quantification and formula confirmation of VOC in real time. J. Am. Soc. Mass Spectrom. 21, 1037–1044 (2010)
Hansel, A., Jordan, A., Holzinger, R., Prazeller, P., Vogel, W., Lindinger, W.: Proton transfer reaction mass spectrometry: on-line trace gas analysis at the ppb level. Int. J. Mass Spectrom. 149, 609–619 (1995)
Biasioli, F., Yeretzian, C., Gasperi, F., Maerk, T.D.: PTR-MS monitoring of VOCs and BVOCs in food science and technology. Trac-Trends Anal. Chem. 30, 968–977 (2011)
Szymanska, E., Brown, P.A., Ziere, A., Martins, S., Batenburg, M., Harren, F.J.M., Buydens, L.M.C.: Comprehensive data scientific procedure for enhanced analysis and interpretation of real-time breath measurements in in vivo aroma-release studies. Anal. Chem. 87, 10338–10345 (2015)
Righettoni, M., Tricoli, A., Gass, S., Schmid, A., Amann, A., Pratsinis, S.E.: Breath acetone monitoring by portable Si:WO3 gas sensors. Anal. Chim. Acta 738, 69–75 (2012)
Samudrala, D., Lammers, G., Mandon, J., Blanchet, L., Schreuder, T.H.A., Hopman, M.T., Harren, F.J.M., Tappy, L., Cristescu, S.M.: Breath acetone to monitor life style interventions in field conditions: an exploratory study. Obesity 22, 980–983 (2014)
Zou, X., Kang, M., Li, A.Y., Shen, C.Y., Chu, Y.N.: Spray inlet proton transfer reaction mass spectrometry (SI-PTR-MS) for rapid and sensitive online monitoring of benzene in water. Anal. Chem. 88, 3144–3148 (2016)
Samudrala, D., Brown, P.A., Mandon, J., Cristescu, S.M., Harren, F.J.M.: Optimization and sensitive detection of sulfur compounds emitted from plants using proton transfer reaction mass spectrometry. Int. J. Mass Spectrom. 386, 6–14 (2015)
Shen, C.Y., Li, J.Q., Han, H.Y., Wang, H.M., Jiang, H.H., Chu, Y.N.: Triacetone triperoxide detection using low reduced-field proton transfer reaction mass spectrometer. Int. J. Mass Spectrom. 285, 100–103 (2009)
Kang, M., Zou, X., Lu, Y., Wang, H.M., Shen, C.Y., Jiang, H.H., Chu, Y.N.: Application of a self-developed proton transfer reaction-mass spectrometer to on-line monitoring trace volatile organic compounds in ambient air. Chem. Res. Chin. Univ. 32, 565–569 (2016)
Sulzer, P., Agarwal, B., Juerschik, S., Lanza, M., Jordan, A., Hartungen, E., Hanel, G., Maerk, L., Maerk, T.D., Gonzalez-Mendez, R., Watts, P., Mayhew, C.A.: Applications of switching reagent ions in proton transfer reaction mass spectrometric instruments for the improved selectivity of explosive compounds. Int. J. Mass Spectrom. 354, 123–128 (2013)
Shen, C.Y., Li, J.Q., Wang, H.Z., Wang, Y.J., Wang, H.M., Huang, C.Q., Li, H., Liu, S., Chu, Y.N.: Proton transfer reaction mass spectrometer with multi-reagent ions. Chem. J. Chin. Univ. 33, 263–267 (2012)
Dunne, E., Galbally, I.E., Lawson, S., Patti, A.: Interference in the PTR-MS measurement of acetonitrile at m/z 42 in polluted urban air—a study using switchable reagent ion PTR-MS. Int. J. Mass Spectrom. 319, 40–47 (2012)
Knighton, W.B., Fortner, E.C., Herndon, S.C., Wood, E.C., Miake-Lye, R.C.: Adaptation of a proton transfer reaction mass spectrometer instrument to employ NO+ as reagent ion for the detection of 1,3-butadiene in the ambient atmosphere. Rapid Commun. Mass Spectrom. 23, 3301–3308 (2009)
Spanel, P., Ji, Y.F., Smith, D.: SIFT studies of the reactions of H3O+, NO+, and O-2(+) with a series of aldehydes and ketones. Int. J. Mass Spectrom. 165, 25–37 (1997)
Karl, T., Hansel, A., Cappellin, L., Kaser, L., Herdlinger-Blatt, I., Jud, W.: Selective measurements of isoprene and 2-methyl-3-buten-2-ol based on NO+ ionization mass spectrometry. Atmos. Chem. Phys. 12, 11877–11884 (2012)
Smit, A.L.C., Field, F.H.: Gaseous anion chemistry. formation and reactions of OH-; reactions of anions with N20; OH-negative chemical ionization. J. Am. Chem. Soc. 99, 6471–6483 (1977)
Custer, T.G., Kato, S., Fall, R., Bierbaum, V.A.: Negative-ion CIMS: analysis of volatile leaf wound compounds including HCN. Int. J. Mass Spectrom. 223, 427–446 (2003)
Shen, C.Y., Niu, W.Q., Huang, C.Q., Xia, L., Lu, Y., Wang, S.L., Wang, H.Z., Jiang, H.H., Chu, Y.N.: Proton-extraction-reaction mass spectrometry (PER-MS) for monitoring organic and inorganic compounds. Int. J. Mass Spectrom. 371, 36–41 (2014)
Zhao, J., Zhang, R.: Proton transfer reaction rate constants between hydronium ion [H3O(+)] and volatile organic compounds. Atmos. Environ. 38, 2177–2185 (2004)
Baasandorj, M., Millet, D.B., Hu, L., Mitroo, D., Williams, B.J.: Measuring acetic and formic acid by proton-transfer-reaction mass spectrometry: sensitivity, humidity, dependence, and quantifying interferences. Atmos. Meas. Tech. 8, 1303–1321 (2015)
Inomata, S., Tanimoto, H.: Differentiation of isomeric compounds by two-stage proton transfer reaction time-of-flight mass spectrometry. J. Am. Soc. Mass Spectrom. 19, 325–331 (2008)
Warneke, C., de Gouw, J.A., Lovejoy, E.R., Murphy, P.C., Kuster, W.C., Fall, R.: Development of proton-transfer ion trap-mass spectrometry: on-line detection and identification of volatile organic compounds in air. J. Am. Soc. Mass Spectrom. 16, 1316–1324 (2005)
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (21577145, 21477132, 81401756), National Key R&D Program of China (2016YFC0200200, 2015BAI01B04), Science and Technology Service Network Initiative, Chinese Academy of Sciences (KFJ-SW-STS-161), Anhui Provincial Program for Science and Technology Development, China (1604d0802001), and Innovation Program of the Development Foundation of the Hefei Center for Physical Science and Technology, China (2014FXCX007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pan, Y., Zhang, Q., Zhou, W. et al. Detection of Ketones by a Novel Technology: Dipolar Proton Transfer Reaction Mass Spectrometry (DP-PTR-MS). J. Am. Soc. Mass Spectrom. 28, 873–879 (2017). https://doi.org/10.1007/s13361-017-1638-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-017-1638-7