Abstract
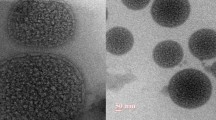
Pulmonary arterial hypertension (PAH) is the increase in mean pulmonary arterial pressure (> 25 mmHg). The development of the non-reversible plexiform lesions on the arterial walls of the pulmonary arteries has evolved as the reason to increase the pressure. The current treatments are directed towards the vasodilation of the pulmonary arteries via the endothelin, prostacyclin, and NO pathways which provides symptomatic relief. Deeper understanding of the disease leads to the various pathophysiological targets that play an important role in the development of PAH. Out of these, the angiogenetic mechanism of the pulmonary arterial smooth muscle cells has been proved to play an important role in PAH. Targeted therapies by anti-proliferative drugs may lead to the efficient treatment strategies to the root cause of PAH. Erlotinib, a receptor tyrosine kinase inhibitor, which acts on the epidermal growth factor receptor (EGFR), has shown promising results in clinical trials of PAH. The objective of the work has been the development of liposomal formulation of anti-proliferative drug, erlotinib HCl, via Quality by Design (QbD) approach. The liposomal formulation was developed using thin-film hydration technique and characterised for various physicochemical parameters, like particle size, % entrapment efficiency, DSC, FTIR, pXRD, and TEM. In the drug release study, the formulation showed sustained release of erlotinib over 24 h in simulated lung fluid pH 7.4. This developed formulation was evaluated in zebrafish tail fin regeneration assay for its anti-angiogenetic activity. The liposomal formulation inhibited the tail fin regeneration for 14 days indicating anti-angiogenetic activity.













Similar content being viewed by others
References
Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014;19(4):558–73.
George MG, Schieb LJ, Ayala C, Talwalkar A, Levant S. Pulmonary hypertension surveillance United States, 2001 to 2010. Chest. 2014;146(2):476–807.
Duarte JD, Hanson RL, Machado RF. Pharmacologic treatments for pulmonary hypertension: exploring pharmacogenomics. Futur Cardiol. 2013;9(3):335–49.
Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51(16):1527–38.
McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. J Am Coll Cardiol. 2015;65(18):1976–97.
McLaughlin VVMD, McGoon MDMD. Pulmonary arterial hypertension. Circ. 2006;114:1417–31.
Smythe W, Mcilleron H, Merle C, Horton J, Smith P, Simonsson, USH. A semi-mechanistic pharmacokinetic auto-induction model for the characterisation of rifampicin pharmacokinetics in African pulmonary tuberculosis infected adults. Poster Abstracts page 2010, 39.
Frumkin LR. The pharmacological treatment of pulmonary arterial hypertension. Pharmacol Rev. 2012;64(3):583–620.
Sakao S, Tatsumi K, Voelkel NF. Reversible or irreversible remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2010;43(6):629–34.
Vaidya B, Gupta V. Novel therapeutic approaches for pulmonary arterial hypertension: unique molecular targets to site-specific drug delivery. J Control Release. 2015;211:118–33.
Galie N, Ghofrani A-H. New horizons in pulmonary arterial hypertension therapies. Eur Respir Rev. 2013;22(130):503–14.
Chakraborty C, Hsu CH, Wen ZH, Lin CS, Agoramoorthy G. Zebrafish: a complete animal model for in vivo drug discovery and development. Curr Drug Metab. 2009;10(2):116–24.
Rubinstein AL. Zebrafish: from disease modeling to drug discovery. Curr Opin Drug Di De. 2003;6:218–23.
Chávez MN, Aedo G, Fierro FA, Allende ML, Egaña JT. Zebrafish as an emerging model organism to study angiogenesis in development and regeneration. Front Physiol. 2016;7(MAR):1–15.
Xu H, He C, Liu Y, Jiang J, Ma T. Novel therapeutic modalities and drug delivery—erlotinib liposomes modified with galactosylated lipid: in vitro and in vivo investigations. Artif Cell Nanomed B. 2018;46(8):1902–7.
Nimmano N, Somavarapu S, Taylor KM. Aerosol characterisation of nebulised liposomes co-loaded with erlotinib and genistein using an abbreviated cascade impactor method. Int J Pharm. 2018;542(1–2):8–17.
Pitsiou G, Zarogoulidis P, Petridis D, Kioumis I, Lampaki S, Organtzis J, et al. Inhaled tyrosine kinase inhibitors for pulmonary hypertension: a possible future treatment. Drug Des Dev Ther. 2014;8:1753–63.
Xu X, Khan MA, Burgess DJ. A quality by design (QbD) case study on liposomes containing hydrophilic API: II. Screening of critical variables, and establishment of design space at laboratory scale. Int J Pharm. 2012;423(2):543–53.
Sylvester B, Porfire A, Muntean DM, Vlase L, Lupuţ L, Licarete E, et al. Optimization of prednisolone-loaded long-circulating liposomes via application of quality by design (QbD) approach. J Lipos Res. 2018;28(1):49–61.
ICH. Pharmaceutical development Q8. ICH Harmon Tripart Guidel. 2009;8(August):1–28.
Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56(6):588–99.
ICH Expert Working Group. Quality risk management Q9. ICH Harmon Tripart Guidel. 2005;(November):1–23.
Roy B, Guha P, Bhattarai R, Nahak P, Karmakar G, Chettri P, et al. Influence of lipid composition, pH, and temperature on physicochemical properties of liposomes with curcumin as model drug. J Oleo Sci. 2016;65(5):399–411.
Mahmoud M, Labiba N, Khalil EK, Khalafallah N. Effect of various formulation variables on the encapsulation and stability of dibucaine base in multilamellar vesicles. Acta Pol Pharm Drug Res. 2005;62(5):369–79.
Kulkarni SB, Betageri GV, Singh M. Factors affecting microencapsulation of drugs in liposomes. J Microencapsul. 1995;12(3):229–46.
Makrilia N, Lappa T, Xyla V, Nikolaidis I, Syrigos K. The role of angiogenesis in solid tumours: an overview. Eur J Intern Med. 2009;20(7):663–71.
Poss KD, Keating MT, Nechiporuk A. Tales of regeneration in zebrafish. Dev Dynam. 2003;226(2):202–10.
Schuermann A, Helker CSM, Herzog W. Angiogenesis in zebrafish. Semin Cell Dev Biol. 2014;31:106–14.
Eyries M, Siegfried G, Ciumas M, Montagne K, Agrapart M, Lebrin F, et al. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res. 2008;103(4):432–40.
Rathinasamy VS, Paneerselvan N, Jagadeeshan S, Malathi R. Hypoxia induced angiogenesis and upregulation of VEGF: an in vivo study using zebrafish model. Int J Sci Eng Res. 2015;6(6):831–9.
Ethical approval and animal care and use
All institutional and national guidelines for care and use of laboratory animals were followed.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Dhoble, S., Patravale, V. Development of anti-angiogenic erlotinib liposomal formulation for pulmonary hypertension: a QbD approach. Drug Deliv. and Transl. Res. 9, 980–996 (2019). https://doi.org/10.1007/s13346-019-00641-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-019-00641-2