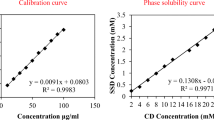
Abstract
Staphylococcus aureus (SA) and methicillin-resistant Staphylococcus aureus (MRSA) have been a major cause of morbidity in thermally injured patients. The skin barrier gets disrupted and loss of immunity further makes burn sites an easy target for bacterial colonization. In the current study, combined potential of lipid-polymer hybrid nanoparticles (LPHNs) with fusidic acid was explored as a promising strategy toward combating resistant bacteria in burn wound infection sites. The developed systems exhibited particle size (310.56 ± 5.22 nm), zeta potential (24.3 ± 4.18 mV) and entrapment efficiency (78.56 ± 3.56%) with a spherical shape. The hybrid nanoparticles were further gelled into carbopol and demonstrated better permeation (76.53 ± 1.55%) and retention characteristics (56.41 ± 4.67%) as compared to the conventional formulation. The topical delivery of FA into the skin layers by FA-LPHN gel was found to be significantly higher (p < 0.05) compared to FA-CC. The in vivo potential was further assessed in murine burn wound model inflicted with MRSA 33591 bacterium with the determination of parameters like bacterial burden, wound contraction, morphological and histopathological examination of wounds. The bacterial count decreased drastically in FA-LPHN gel group (5.22 log CFU/mL) on day 3 with significant difference in comparison to FA-CC. The wound size reduction in FA-LPHN gel (68.70 ± 3.65%) was higher as compared to FA-CC (73.30 ± 4.23%) and control groups (83.30 ± 4.40%) on day 5. The current study presents a safe and effective formulation strategy for the treatment of MRSA-infected burn wounds by providing moist environment and prevention from bacterial infection.






Similar content being viewed by others
References
Church D, Elsayed S, Reid O, Winston R, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19:403–34.
Weinstein RA, Mayhall CG. The epidemiology of burn wound infections: then and now. Clin Infect Dis. 2003;37:543–50.
Mofazzal Jahromi MA, Sahandi Zangabad P, Moosavi Basri SM, Sahandi Zangabad K, Ghamarypour A, Aref AR, et al. Nanomedicine and advanced technologies for burns: preventing infection and facilitating wound healing. Adv Drug Deliv Rev. 2018;123:33–64.
Haisma EM, de Breij A, Chan H, van Dissel JT, Drijfhout JW, Hiemstra PS, et al. LL-37-derived peptides eradicate multidrug-resistant Staphylococcus aureus from thermally wounded human skin equivalents. Antimicrob Agents Chemother. 2014;58:4411–9.
Branski LK, Al-Mousawi A, Rivero H, Jeschke MG, Sanford AP, Herndon DN. Emerging infections in burns. Surg Infect. 2009;10:389–97.
Schlievert PM, Strandberg KL, Lin YC, Peterson ML, Leung DY. Secreted virulence factor comparison between methicillin-resistant and methicillin-sensitive Staphylococcus aureus, and its relevance to atopic dermatitis. J Allergy Clin Immunol. 2010;125:39–49.
O'Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev. 2004;17:218–34.
Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9.
Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–42.
Foster TJ. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol Rev. 2017;41:430–49.
Upton A, Lang S, Heffernan H. Mupirocin and Staphylococcus aureus: a recent paradigm of emerging antibiotic resistance. J Antimicrob Chemother. 2003;51:613–7.
Kalita S, Kandimalla R, Devi B, Kalita B, Kalita K, Deka M, et al. Dual delivery of chloramphenicol and essential oil by poly-?-caprolactone-Pluronic nanocapsules to treat MRSA-Candida co-infected chronic burn wounds. RSC Adv. 2017;7:1749–58.
Xu F, Weng B, Gilkerson R, Materon LA, Lozano K. Development of tannic acid/chitosan/pullulan composite nanofibers from aqueous solution for potential applications as wound dressing. Carbohydr Polym. 2015;115:16–24.
Anjum S, Gupta A, Sharma D, Gautam D, Bhan S, Sharma A, et al. Development of novel wound care systems based on nanosilver nanohydrogels of polymethacrylic acid with Aloe vera and curcumin. Mater Sci Eng C. 2016;64:157–66.
Sanchez DA, Schairer D, Tuckman-Vernon C, Chouake J, Kutner A, Makdisi J, et al. Amphotericin B releasing nanoparticle topical treatment of <em>Candida spp.</em> in the setting of a burn wound. Nanomedicine. 2014;10:269–77.
Fan Y, Ciotti S, Cao Z, Eisma R, Baker J, Wang SH. Screening of nanoemulsion formulations and identification of NB-201 as an effective topical antimicrobial for Staphylococcus aureus in a mouse model of infected wounds. Mil Med Res. 2016;181:259–64.
Verbist L. The antimicrobial activity of fusidic acid. J Antimicrob Chemother. 1990;25:1–5.
Howden BP, Grayson ML. Dumb and dumber-the potential waste of a useful antistaphylococcal agent: emerging Fusidic acid resistance in Staphylococcus aureus. Clin Infect Dis. 2006;42:394–400.
Wadhwa S, Singh B, Sharma G, Raza K, Katare OP. Liposomal fusidic acid as a potential delivery system: a new paradigm in the treatment of chronic plaque psoriasis. Drug Deliv. 2016;23:1204–13.
Sharma G, Thakur K, Raza K, Singh B, Katare OP. Nanostructured lipid carriers: a new paradigm in topical delivery for dermal and transdermal applications. Crit Rev Ther Drug Carrier Syst. 2017;34:355–86.
Thakur K, Sharma G, Singh B, Chhibber S, Katare OP. Current state of nanomedicines in the treatment of topical infectious disorders. Recent Pat Antiinfect Drug Discov. 2018. https://doi.org/10.2174/1574891X13666180529103804.
Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;65:1803–15.
Sharma G, Goyal H, Thakur K, Raza K, Katare OP. Novel elastic membrane vesicles (EMVs) and ethosomes-mediated effective topical delivery of aceclofenac: a new therapeutic approach for pain and inflammation. Drug Deliv. 2016;23:3135–45.
Sharma G, Kaur M, Raza K, Thakur K, Katare OP. Aceclofenac-β-cyclodextrin-vesicles: a dual carrier approach for skin with enhanced stability, efficacy and dermatokinetic profile. RSC Adv. 2016;6:20713–27.
Jain A, Sharma G, Ghoshal G, Kesharwani P, Singh B, Shivhare US, et al. Lycopene loaded whey protein isolate nanoparticles: an innovative endeavor for enhanced bioavailability of lycopene and anti-cancer activity. Int J Pharm. 2018;546:97–105. https://doi.org/10.1016/j.ijpharm.2018.04.061.
Jain A, Sharma G, Kushwah V, Ghoshal G, Jain A, Singh B, et al. Beta carotene-loaded zein nanoparticles to improve the biopharmaceutical attributes and to abolish the toxicity of methotrexate: a preclinical study for breast cancer. Artif Cells Nanomed Biotechnol. 2018;23:1–11.
Garg NK, Tyagi RK, Sharma G, Jain A, Singh B, Jain S, et al. Functionalized lipid-polymer hybrid nanoparticles mediated codelivery of methotrexate and aceclofenac: a synergistic effect in breast cancer with improved pharmacokinetics attributes. Mol Pharm. 2017;14:1883–97. https://doi.org/10.1021/acs.molpharmaceut.6b01148.
Thakur K, Sharma G, Singh B, Chhibber S, Patil AB, Katare OP. Chitosan-tailored lipidic nanoconstructs of fusidic acid as promising vehicle for wound infections: an explorative study. Int J Biol Macromol. 2018;115:1012–25.
Jain A, Sharma G, Kushwah V, Garg NK, Kesharwani P, Ghoshal G, et al. Methotrexate and beta-carotene loaded-lipid polymer hybrid nanoparticles: a preclinical study for breast cancer. Nanomedicine (Lond). 2017;12:1851–72.
Forier K, Raemdonck K, De Smedt SC, Demeester J, Coenye T, Braeckmans K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J Control Release. 2014;190:607–23.
Ueno H, Mori T, Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev. 2001;52:105–15.
Kong M, Chen XG, Xing K, Park HJ. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol. 2010;144:51–63.
Thakur K, Sharma G, Singh B, Jain A, Tyagi R, Chhibber S, et al. Cationic-bilayered nanoemulsion of fusidic acid: an investigation on eradication of methicillin-resistant Staphylococcus aureus 33591 infection in burn wound. Nanomedicine (Lond). 2018;13:825–47.
Jain A, Thakur K, Sharma G, Kush P, Jain UK. Fabrication, characterization and cytotoxicity studies of ionically cross-linked docetaxel loaded chitosan nanoparticles. Carbohydr Polym. 2016;137:65–74.
Yadav NK, Nanda S, Sharma G, Katare OP. Systematically optimized coenzyme q10-loaded novel proniosomal formulation for treatment of photo-induced aging in mice: characterization, biocompatibility studies, biochemical estimations and anti-aging evaluation. J Drug Target. 2016;24:257–71. https://doi.org/10.3109/1061186X.2015.77845.
Yadav NK, Nanda S, Sharma G, Katare OP. Systematically optimized ketoprofen-loaded novel proniosomal formulation for periodontitis: in vitro characterization and in vivo pharmacodynamic evaluation. AAPS PharmSciTech. 2017;18:1863–80. https://doi.org/10.1208/s12249-016-0665-1.
Jain A, Sharma G, Kushwah V, Thakur K, Ghoshal G, Singh B, et al. Fabrication and functional attributes of lipidic nanoconstructs of lycopene: an innovative Endeavour for enhanced cytotoxicity in MCF-7 breast cancer cells. Colloids Surf B Biointerfaces. 2017;152:482–91.
Sharma G, Saini MK, Thakur K, Kapil N, Garg NK, Raza K, et al. Aceclofenac cocrystal nanoliposomes for rheumatoid arthritis with better dermatokinetic attributes: a preclinical study. Nanomedicine(Lond). 2017;12:615–38.
Sharma G, Kamboj S, Thakur K, Negi P, Raza K, Katare OP. Delivery of Thermoresponsive-tailored mixed micellar nanogel of lidocaine and prilocaine with improved dermatokinetic profile and therapeutic efficacy in topical anaesthesia. AAPS PharmSciTech. 2017;18:790–802.
Sharma G, Devi N, Thakur K, Jain A, Katare OP. Lanolin-based organogel of salicylic acid: evidences of better dermatokinetic profile in imiquimod-induced keratolytic therapy in BALB/c mice model. Drug Deliv Transl Res. 2018;8:398–413.
Abioye AO, Issah S, Kola-Mustapha AT. Ex vivo skin permeation and retention studies on chitosan “ibuprofen” gellan ternary nanogel prepared by in situ ionic gelation technique: a tool for controlled transdermal delivery of ibuprofen. Int J Pharm. 2015;490:112–30.
Nirbhavane P, Sharma G, Singh B, Khuller GK, Goni VG, Patil AB, et al. Preclinical explorative assessment of celecoxib-based biocompatible lipidic nanocarriers for the management of CFA-induced rheumatoid arthritis in Wistar rats. AAPS PharmSciTech. 2018;24:018–1148.
Negi P, Singh B, Sharma G, Beg S, Katare OP. Biocompatible lidocaine and prilocaine loaded-nanoemulsion system for enhanced percutaneous absorption: QbD-based optimisation, dermatokinetics and in vivo evaluation. J Microencapsul. 2015;32:419–31.
Sharma G, Dhankar G, Thakur K, Raza K, Katare OP. Benzyl benzoate-loaded microemulsion for topical applications: enhanced dermatokinetic profile and better delivery promises. AAPS PharmSciTech. 2016;17:1221–31.
Ito K, Saito A, Fujie T, Nishiwaki K, Miyazaki H, Kinoshita M, et al. Sustainable antimicrobial effect of silver sulfadiazine-loaded nanosheets on infection in a mouse model of partial-thickness burn injury. Acta Biomater. 2015;24:87–95.
Chhibber T, Wadhwa S, Chadha P, Sharma G, Katare OP. Phospholipid structured microemulsion as effective carrier system with potential in methicillin sensitive Staphylococcus aureus (MSSA) involved burn wound infection. J Drug Target. 2015;23:943–52.
Simonetti O, Lucarini G, Orlando F, Pierpaoli E, Ghiselli R, Provinciali M, et al. Role of Daptomycin on burn wound healing in an animal methicillin-resistant Staphylococcus aureus infection model. Antimicrob Agents Chemother. 2017;61:e00606–17.
Adhikari RP, Thompson CD, Aman MJ, Lee JC. Protective efficacy of a novel alpha hemolysin subunit vaccine (AT62) against Staphylococcus aureus skin and soft tissue infections. Vaccine. 2016;34:6402–7.
El-Refaie WM, Elnaggar YSR, El-Massik MA, Abdallah OY. Novel curcumin-loaded gel-core hyaluosomes with promising burn-wound healing potential: development, in-vitro appraisal and in-vivo studies. Int J Pharm. 2015;486:88–98.
Fang Y, Gong SJ, Xu YH, Hambly BD, Bao S. Impaired cutaneous wound healing in granulocyte/macrophage colony-stimulating factor knockout mice. Br J Dermatol. 2007;157:458–65.
Zhang H, Chen J, Cen Y. Burn wound healing potential of a polysaccharide from Sanguisorba officinalis L. in mice. Int J Biol Macromol. 2018;112:862–7.
Wills ED. Mechanisms of lipid peroxide formation in tissues role of metals and haematin proteins in the catalysis of the oxidation of unsaturated fatty acids. Biochim Biophys Acta. 1965;98:238–51.
Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95.
Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–40.
Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78.
Damian F, Blaton N, Naesens L, Balzarini J, Kinget R, Augustijns P, et al. Physicochemical characterization of solid dispersions of the antiviral agent UC-781 with polyethylene glycol 6000 and Gelucire 44/14. Eur J Pharm Sci. 2000;10:311–22.
Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery: a review of the state of the art. Europ J Pharm Biopharm. 2000;50:161–77.
Hadinoto K, Sundaresan A, Cheow WS. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: a review. Eur J Pharm Biopharm. 2013;85:427–43.
Mizrahy S, Peer D. Polysaccharides as building blocks for nanotherapeutics. Chem Soc Rev. 2012;41:2623–40.
Philibert T, Lee BH, Fabien N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl Biochem Biotechnol. 2017;181:1314–37.
Tran TH, Nguyen TD, Poudel BK, Nguyen HT, Kim JO, Yong CS, et al. Development and evaluation of artesunate-loaded chitosan-coated lipid nanocapsule as a potential drug delivery system against breast cancer. AAPS PharmSciTech. 2015;16:1307–16.
Jain A, Thakur K, Kush P, Jain UK. Docetaxel loaded chitosan nanoparticles: formulation, characterization and cytotoxicity studies. Int J Biol Macromol. 2014;69:546–53.
Qi L, Xu Z, Jiang X, Hu C, Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res. 2004;339:2693–700.
Desai PR, Marepally S, Patel AR, Voshavar C, Chaudhuri A, Singh M. Topical delivery of anti-TNFalpha siRNA and capsaicin via novel lipid-polymer hybrid nanoparticles efficiently inhibits skin inflammation in vivo. J Control Release. 2013;170:51–63.
Caon T, Porto LC, Granada A, Tagliari MP, Silva MA, Simoes CM, et al. Chitosan-decorated polystyrene-b-poly(acrylic acid) polymersomes as novel carriers for topical delivery of finasteride. Eur J Pharm Sci. 2014;52:165–72.
Dorrani M, Kaul M, Parhi A, LaVoie EJ, Pilch DS, Michniak-Kohn B. TXA497 as a topical antibacterial agent: comparative antistaphylococcal, skin deposition, and skin permeation studies with mupirocin. Int J Pharm. 2014;476:199–204.
Wang J, Zhang L, Chi H, Wang S. An alternative choice of lidocaine-loaded liposomes: lidocaine-loaded lipid-polymer hybrid nanoparticles for local anesthetic therapy. Drug Deliv. 2016;23:1254–60.
Dave V, Kushwaha K, Yadav RB, Agrawal U. Hybrid nanoparticles for the topical delivery of norfloxacin for the effective treatment of bacterial infection produced after burn. J Microencapsul. 2017;34:351–65.
Kong HH, Segre JA. Skin microbiome: looking back to move forward. J Invest Dermatol. 2012;132:933–9.
El Maghraby GM, Barry BW, Williams AC. Liposomes and skin: from drug delivery to model membranes. Eur J Pharm Sci. 2008;34:203–22.
Zakir F, Vaidya B, Goyal AK, Malik B, Vyas SP. Development and characterization of oleic acid vesicles for the topical delivery of fluconazole. Drug Deliv. 2010;17:238–48.
Luo Y, Teng Z, Li Y, Wang Q. Solid lipid nanoparticles for oral drug delivery: chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr Polym. 2015;122:221–9.
Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin wound healing: an update on the current knowledge and concepts. Eur Surg Res. 2017;58:81–94.
Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen. 2014;22:569–78.
Acknowledgements
The authors acknowledge Sun Pharma Pvt. Ltd., Goa, India and M/s Phospholipid GmbH, (Germany) for generous gift samples of drug molecule and phospholipids. The first author, Kanika Thakur acknowledges the Department of Science & Technology, New Delhi, India, for financial assistance as DST INSPIRE SRF (IF140162).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thakur, K., Sharma, G., Singh, B. et al. Nano-engineered lipid-polymer hybrid nanoparticles of fusidic acid: an investigative study on dermatokinetics profile and MRSA-infected burn wound model. Drug Deliv. and Transl. Res. 9, 748–763 (2019). https://doi.org/10.1007/s13346-019-00616-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-019-00616-3