Abstract
Objective
In type 2 diabetes, the significant pathological change in pancreatic islets is amyloid deposits. Its major component is islet amyloid polypeptide (IAPP). The objective of this study was to evaluate the possibility that the effect of the IAPP genotype on β-cell dysfunction in type 2 diabetes is modified by variations in plasma glucose levels.
Methods
Participants from the Toon Genome Study underwent a 75 g OGTT for the diagnosis of glucose tolerance and the evaluation of insulin secretion. We examined the effect of a SNP, rs77397980, on β-cell function by analyzing an interaction (statistics) between the IAPP genotype and AUC glucose.
Results
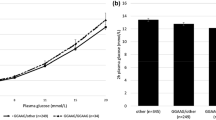
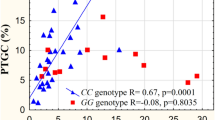
The ratio of the C-allele carriers was essentially the same among subjects with normal glucose tolerance, impaired glucose tolerance and diabetes. In subjects with diabetes, along with an increase in AUC glucose, fasting insulin remained constant in the T/T homozygotes and appeared to decrease in the C-allele carriers. A homeostasis model assessment (HOMA)-IR appeared to be increased in the former and decreased in the latter. In subjects with diabetes stratified into cases with higher AUC glucose than the median, fasting insulin and HOMA-IR were lower in the C-allele carriers than in the T/T homozygotes. An interaction between the IAPP genotype and AUC glucose was indicated in the effect on HOMA-IR.
Conclusions
The possibility that the association between IAPP genotype and basal insulin level is modified by variation in plasma glucose, resulting in a decreased basal insulin in type 2 diabetes, cannot be excluded.


Similar content being viewed by others
References
Halban PA, Polonsky KS, Bowden DW, et al. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care. 2014;37:1751–8.
Opie EL. The relation of diabetes mellitus to lesion of the pancreas. Hyaline degeneration of the islands of Langerhans. J Exp Med. 1901;5:527–40.
Ohsawa H, Kanatsuka A, Mizuno Y, et al. Islet amyloid polypeptide-derived amyloid deposition increases along with the duration of type 2 diabetes mellitus. Diabetes Res Clin Pract. 1992;15:17–22.
Westermark P, Wernstedt C, Wilander E, Hayden DW, O’Brien TD, Johnson KH. Amyloid fibrils in human insulinoma and islets Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci USA. 1987;84:3881–5.
Cooper GJS, Willis AC, Clark A, Turner RC, Sim RB, Reid KBM. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA. 1987;84:8628–32.
Mosselman S, Hoppener JWM, Lips CJM, Jansz HS. The complete islet amyloid polypeptide precursor is encoded by two exons. FEBS Lett. 1989;247:154–8.
Sanke T, Bell GI, Sample C, Rubenstein AH, Steiner DF. An islet amyloid peptide is derived from an 89-amino acid precursor by proteolytic processing. J Biol Chem. 1988;263:17243–6.
Kanatsuka A, Makino H, Ohsawa H, et al. Secretion of islet amyloid polypeptide in response to glucose. FEBS Lett. 1989;259:199–201.
Ogawa A, Harris V, McCorkle SK, Unger RH, Luskey KL. Amylin secretion from the rat pancreas and its selective loss after streptozotocin treatment. J Clin Invest. 1990;85:973–6.
Kanatsuka A, Makino H, Yamaguchi T, et al. Islet amyloid polypeptide/amylin in pancreatic β-cell line derived from transgenic mouse insulinoma. Diabetes. 1992;41:1409–14.
Ohsawa H, Kanatsuka A, Yamaguchi T, Makino H, Yoshida S. Islet amyloid polypeptide inhibits glucose-stimulated insulin secretion from isolated rat pancreatic islets. Biochem Biophys Res Commun. 1989;160:961–7.
Degano P, Silvestre RA, Salas M, Peiro E. Amylin inhibits glucose-induced insulin secretion in a dose-dependent manner. Study in the perfused rat pancreas. Regul Pept. 1993;43:91–6.
Yagui K, Yamaguchi T, Kanatsuka A, et al. Formation of islet amyloid fibrils in beta-secretory granules of transgenic mice expressing human islet amyloid polypeptide/amylin. Eur J Endocrinol. 1995;132:487–96.
Tokuyama T, Yagui K, Yamaguchi T, et al. Expression of human islet amyloid polypeptide/amylin impairs insulin secretion in mouse pancreatic β cells. Metabolism. 1997;46:1044–51.
Janson J, Soeller WC, Roche PC, et al. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci USA. 1996;93:7283–8.
Cook JTE, Patel PP, Clark A, et al. Non-linkage of the islet amyloid polypeptide gene with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1991;34:103–8.
Nishi M, Bell GI, Steiner DF. Islet amyloid polypeptide (amylin): no evidence of an abnormal precursor sequence in 25 type 2 (non-insulin dependent) diabetic patients. Diabetologia. 1990;33:628–30.
Zee RYL, Pulido-Perez P, Perez-Fuentes R, Ridker PM, Chasman DI, Romero JR. Islet amyloid polypeptide gene variation (IAPP) and the risk of incident type 2 diabetes mellitus: the Women’s Genome Health Study. Clin Chim Acta. 2011;11:785–7.
Kanatsuka A, Kou S, Makino H. IAPP/amylin and β-cell failure: implication of the risk factors of type 2 diabetes. Diabetol Int. 2018;9:143–57.
Lindstroem P. The physiology of obese-hyperglycemic mice (ob/ob mice). Sci World J. 2007;7:666–85.
Hoppener JWM, Oosterwijk C, Nieuwenhuis MG, et al. Extensive islet amyloid formation is induced by development of type II diabetes mellitus and contributes to its progression: pathogenesis of diabetes in a mouse model. Diabetologia. 1999;42:427–34.
Soeller WC, Janson J, Hart SE, et al. Islet amyloid-associated diabetes in obese Avy/a mice expressing human islet amyloid polypeptide. Diabetes. 1998;47:743–50.
Yen TT, Gill AM, Frigeri LG, Barsh GS, Wolff GL. Obesity, diabetes, and neoplasia in yellow A(vy)/- mice: ectopic expression of the agouti gene. FASEB J. 1994;8:479–88.
Roostaei T, Nazeri A, Felsky D, et al. for the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Genome-wide interaction study of brain beta-amyloid burden and cognitive impairment in Alzheimer’s disease. Mol Psychiatry. 2017;22:287–95.
Noumi Y, Kawamura R, Tabara Y, et al. An inverse association between serum resistin levels and n-3 polyunsaturated fatty acids intake was strongest in the SNP-420 G/G genotype in the Japanese cohort: the Toon Genome Study. Clin Endocrinol. 2018;88:51–7.
Cox DR. Interaction. Int Stat Rev. 1984;52:1–25.
Expert committee on the diagnosis and classification of diabetes mellitus report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997; 20:1183–97.
The guideline for epidemiology study in Japan. The ministry of health, labor and welfare. June 30, 2003 (Japanese).
Matthews DR, Hosker JR, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Kahn BB. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell. 1998;92:593–6.
Polonsky KS. The β-cell in diabetes: from molecular genetics to clinical research. Diabetes. 1995;44:706–17.
Mosselman S, Hoppener JWM, de Wit L, Soeller WC, Lips CJM, Jansz HS. IAPP/amylin gene transcriptional control region: evidence for negative regulation. FEBS Lett. 1990;271:33–6.
German MS, Moss LG, Wang JH, Rutter WJ. The insulin and islet amyloid polypeptide genes contain similar cell-specific promoter elements that bind identical beta-cell nuclear complexes. Mol Cell Biol. 1992;12:1777–88.
Gurio T, Ryazantsev S, Huang C-J, et al. Evidence for proteotoxicity in β-cells in type 2 diabetes. Toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway. Am J Pathol. 2010;176:861–9.
Apostolidou M, Jayasinghe SA, Langen R. Structure of & α-helical membrane-bound human islet amyloid polypeptide and its implications for membrane-mediated misfolding. J Biol Chem. 2008;283:17205–10.
Patil AM, Xu S, Sheftic SR, Alexanderescu AT. Dynamic & α-helix structure of micelle-bound human amylin. J Biol Chem. 2009;284:11982–91.
Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115:2047–58.
Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121:2118–25.
Wiebe N, Ye F, Crumley ET, Bello A, Stenvinkel P, Tonelli M. Temporal associations among body mass index, fasting insulin, and systemic inflammation. A systematic review and meta-analysis. JAMA Netw Open. 2021;4:e211263.
Acknowledgements
The authors wish to thank the staffs in the Department of Diabetes and Molecular Genetics, Ehime University Graduate School of Medicine, Ehime, Japan for the support of the Toon Genome Study, the ex-staffs in the Diabetes Research Group, the Second Department of Internal Medicine, Chiba University School of Medicine, Chiba, Japan for the suggestions regarding the conduct of this study, and Dr. Jun Ohashi, Department of Biological Sciences, Graduate School of Science, The University of Tokyo, for the suggestions for statistical analyses.
Funding
This study was supported by JSPS KAKENHI, Health Labor Sciences Research Grant, Grants from Ehime University, and the Japan Diabetes Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest regarding the publication of this paper.
Human rights statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Ehime university hospital and graduate school of medicine, ethics committee, date of approval: 1 April. 2009, approval no. 29-K3, 31-4, and 31-18) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent was obtained from the participants in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Kawamura, R., Tabara, Y., Takata, Y. et al. Association of a SNP in the IAPP gene and hyperglycemia on β-cell dysfunction in type 2 diabetes: the Toon Genome Study. Diabetol Int 13, 201–208 (2022). https://doi.org/10.1007/s13340-021-00523-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-021-00523-4