Abstract
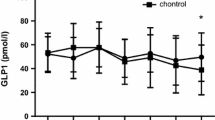
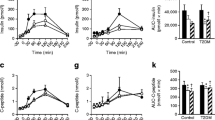
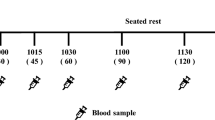
This pilot study aimed to examine the effect of pre-meal tasteless calorie-free gum chewing on post-meal blood levels of glucose, insulin, glucagon, and gastrointestinal hormones. This was an open-label, randomized, 2-sequence, 3-period, 2-treatment crossover trial with a 1:1 allocation. Sixteen Japanese adult male volunteers aged between 30 and 49 years without diagnosed glucose metabolism disorder were enrolled. Ingestion of 200-g cooked rice after 15-min tasteless calorie-free gum chewing (GUM+ treatment) was compared to that without preceding gum chewing (GUM− treatment). Cooked rice was divided into twelve equally sized portions and consumed by chewing each portion 30 times before swallowing. Treatment sessions were separated by an at least 1-week interval and attended after an overnight fast. Circulating levels of glucose, insulin, glucagon, active glucagon-like peptide (GLP)-1 and ghrelin were measured at baseline (before treatment) and 0, 15, 30, 60, and 120 min after completion of the meal ingestion, and the postprandial change from baseline was assessed. As a result, the change in glucose levels at 0 min was significantly lower in the GUM+ treatment than in the GUM− treatment (P = 0.004). Furthermore, the GUM+ treatment demonstrated higher incremental insulin levels at 15 min (P = 0.041) and higher incremental active GLP-1 levels at 30 and 60 min (P = 0.018 and 0.021, respectively); whereas, postprandial glucagon and ghrelin levels were not significantly different. In conclusion, the current pilot study demonstrated that tasteless calorie-free gum chewing before rice eating had a significant but limited impact on the increase of postprandial active GLP-1 levels in male individuals without diagnosed glucose metabolism disorder.


Similar content being viewed by others
References
Li J, Zhang N, Hu L, Li Z, Li R, Li C, et al. Improvement in chewing activity reduces energy intake in one meal and modulates plasma gut hormone concentrations in obese and lean young Chinese men. Am J Clin Nutr. 2011;94:709–16.
Sonoki K, Iwase M, Takata Y, Nakamoto T, Masaki C, Hosokawa R, et al. Effects of thirty-times chewing per bite on secretion of glucagon-like peptide-1 in healthy volunteers and type 2 diabetic patients. Endocr J. 2013;60:311–9.
Zhu Y, Hsu WH, Hollis JH. Increasing the number of masticatory cycles is associated with reduced appetite and altered postprandial plasma concentrations of gut hormones, insulin and glucose. Br J Nutr. 2013;110:384–90.
Xu J, Xiao X, Li Y, Zheng J, Li W, Zhang Q, et al. The effect of gum chewing on blood GLP-1 concentration in fasted, healthy, non-obese men. Endocrine. 2015;50:93–8.
Haneda M, Noda M, Origasa H, Noto H, Yabe D, Fujita Y, Goto A, Kondo T, Araki E (2018) Japanese clinical practice guideline for diabetes 2016. Diabetol Int 9(1):1–45
Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care. 2009;32:2184–6.
Matsuo T, Miyagawa J, Kusunoki Y, Miuchi M, Ikawa T, Akagami T, et al. Postabsorptive hyperglucagonemia in patients with type 2 diabetes mellitus analyzed with a novel enzyme-linked immunosorbent assay. J Diabetes Investig. 2016;7:324–31.
Yabe D, Watanabe K, Sugawara K, Kuwata H, Kitamoto Y, Sugizaki K, et al. Comparison of incretin immunoassays with or without plasma extraction: Incretin secretion in Japanese patients with type 2 diabetes. J Diabetes Investig. 2012;3:70–9.
Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011;54:10–8.
Koopman ADM, Rutters F, Rauh SP, Nijpels G, Holst JJ, Beulens JW, et al. Incretin responses to oral glucose and mixed meal tests and changes in fasting glucose levels during 7 years of follow-up: the Hoorn Meal Study. PLoS ONE. 2018;13:e0191114.
Kim SH, Abbasi F, Lamendola C, Liu A, Ariel D, Schaaf P, et al. Benefits of liraglutide treatment in overweight and obese older individuals with prediabetes. Diabetes Care. 2013;36:3276–82.
Rasmussen MF. The development of oral semaglutide, an oral GLP-1 analog, for the treatment of type 2 diabetes. Diabetol Int. 2020. https://doi.org/10.1007/s13340-019-00423-8.
le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, Van Gaal L, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389:1399–409.
Suzuki H, Fukushima M, Okamoto S, Takahashi O, Shimbo T, Kurose T, et al. Effects of thorough mastication on postprandial plasma glucose concentrations in nonobese Japanese subjects. Metabolism. 2005;54:1593–9.
Ranawana V, Clegg ME, Shafat A, Henry CJ. Postmastication digestion factors influence glycemic variability in humans. Nutr Res. 2011;31:452–9.
Herrmann-Rinke C, Voge A, Hess M, Goke B. Regulation of glucagon-like peptide-1 secretion from rat ileum by neurotransmitters and peptides. J Endocrinol. 1995;147:25–31.
Hansen L, Lampert S, Mineo H, Holst JJ. Neural regulation of glucagon-like peptide-1 secretion in pigs. Am J Physiol Endocrinol Metab. 2004;287:E939–E947947.
Anini Y, Brubaker PL. Muscarinic receptors control glucagon-like peptide 1 secretion by human endocrine L cells. Endocrinology. 2003;144:3244–50.
Ohta M, Ueda T, Sakurai K. Effect of chewing or compressing food on autonomic nervous activity in older adults. Gerodontology. 2017;34:434–40.
Hopman WP, Jansen JB, Rosenbusch G, Lamers CB. Cephalic stimulation of gallbladder contraction in humans: role of cholecystokinin and the cholinergic system. Digestion. 1987;38:197–203.
Ostoft SH, Bagger JI, Hansen T, Hartmann B, Pedersen O, Holst JJ, et al. Postprandial incretin and islet hormone responses and dipeptidyl-peptidase 4 enzymatic activity in patients with maturity onset diabetes of the young. Eur J Endocrinol. 2015;173:205–15.
Idorn T, Knop FK, Jorgensen MB, Christensen M, Holst JJ, Hornum M, et al. Elimination and degradation of glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with end-stage renal disease. J Clin Endocrinol Metab. 2014;99:2457–66.
Smeets PA, Erkner A, de Graaf C. Cephalic phase responses and appetite. Nutr Rev. 2010;68:643–55.
Acknowledgements
The authors acknowledge the assistance of Soiken Inc., Tokyo, Japan, the contract research organization that contributed to the data management and data analysis in the current study.
Funding
The current study, sponsored by the Osaka Association of General Physician, was funded by LOTTE Co., Ltd., Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mitsuyoshi Takahara is a staff member of the endowed chair (Department of Diabetes Care Medicine) which received funds from AstraZeneca K.K., Mitsubishi Tanabe Pharma Co., MSD K.K., Nippon Boehringer Ingelheim Co., Novo Nordisk Pharma, Ono Pharmaceutical Co., and Taisho Toyama Pharmaceutical Co.. Masahiro Fukuda has received honoraria from Mitsubishi Tanabe Pharma Co., MSD K.K., Ono Pharmaceutical Co., Sanofi K.K., and Takeda Pharmaceutical Co., and has received research funding from Astellas Pharma, LOTTE Co., Novo Nordisk Pharma, and Sanofi K.K.. Yuji Matsuzawa declares that he has no conflict of interest. Iichiro Shimomura has received honoraria from Astellas Pharma, Eli Lilly Japan K.K., Kowa Co., Mitsubishi Tanabe Pharma Co., MSD K.K., Nippon Boehringer Ingelheim Co., Novo Nordisk Pharma, Ono Pharmaceutical Co., Sanofi K.K., Sanwa Kagaku Kenkyusho Co., and Takeda Pharmaceutical Co., and has received subsides or donations from Astellas Pharma, AstraZeneca K.K., Daiichi Sankyo Co., Kowa Co., Kyowa Kirin Co., Mitsubishi Tanabe Pharma Co., MSD K.K., Novartis Pharmaceuticals Corp., Novo Nordisk Pharma, Ono pharmaceutical Co., Sanofi K.K., Sumitomo Dainippon Pharma., Takeda Pharmaceutical Co., Teijin Pharma, and Terumo Corporation.
Human rights statement and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and/or with the Helsinki Declaration of 1964 and later versions. Informed consent or substitute for it was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Takahara, M., Fukuda, M., Matsuzawa, Y. et al. Effect of tasteless calorie-free gum chewing before meal on postprandial plasma glucose, insulin, glucagon, and gastrointestinal hormones in Japanese men without diagnosed glucose metabolism disorder: a pilot randomized crossover trial. Diabetol Int 11, 394–402 (2020). https://doi.org/10.1007/s13340-020-00435-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-020-00435-9