Abstract
Aims
The purpose of this study was to clarify the predictive clinical characteristics of therapy switching from sitagliptin to dulaglutide in patients with type 2 diabetes mellitus.
Methods
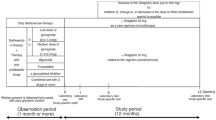
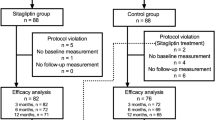
This single-center, open-label, investigator-initiated pilot study was conducted in 40 patients with type 2 diabetes mellitus. The patients, who had been treated with 50 mg sitagliptin daily for at least 6 months were switched to 0.75 mg dulaglutide weekly.
Results
A total of 36 patients could be followed for 24 weeks of treatment with dulaglutide. They were assessed for several clinical parameters before the start of and 24 weeks after the study. Multiple linear regression analysis was used to search for independent predictors of reduction of hemoglobin A1c (HbA1c) levels after 24 weeks of treatment switching from sitagliptin to dulaglutide. Dulaglutide administration for 24 weeks resulted in significant reductions in fasting plasma glucose (FPG), HbA1c, and low-density lipoprotein cholesterol (LDL-C) levels. In addition, baseline HbA1c, FPG, body mass index (BMI), and age were significantly correlated with the change in HbA1c levels (ΔHbA1c). Furthermore, multiple linear regression analysis revealed that BMI and age significantly correlated with ΔHbA1c.
Conclusion
In summary, our prospective 24-week study showed that baseline low BMI and old age are significantly useful in predicting the HbA1c-lowering effect of switching from sitagliptin to dulaglutide.
Trial Registration
UMIN Clinical Trials Registry (UMIN000023245).


Similar content being viewed by others
References
Brunton SA. Hypoglycemic potential of current and emerging pharmacotherapies in type 2 diabetes mellitus. Postgrad Med. 2012;124:74–83.
Morgan CL, Jenkins-Jones S, Evans M, Barnett AH, Poole CD, Currie CJ. Weight change in people with type 2 diabetes: secular trends and the impact of alternative antihyperglycaemic drugs. Diabetes Obes Metab. 2012;14:424–32.
Majima T, Komatsu Y, Doi K, Shigemoto M, Takagi C, Fukao A, Corners J, Nakao K. Safety and efficacy of low-dose pioglitazone (7.5 mg/day) vs. standard-dose pioglitazone (15 mg/day) in Japanese women with type 2 diabetes mellitus. Endocr J. 2006;53:325–30.
Japan Diabetes Society. Treatment Guide for Diabetes 2014–2015 (2014) Available from http://www.fa.kyorin.co.jp/jds/uploads/Treatment_Guide_for_Diabetes_2014-2015.pdf. Accessed 29 June 2017.
Trulicity [Summary of Product Characteristics] Houten, The Netherlands: Eli Lilly and Company, 2014.
Raccah D, Miossec P, Esposito V, Niemoeller E, Cho M, Gerich J. Efficacy and safety of lixisenatide in elderly (≥ 65 years old) and very elderly (≥ 75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev. 2015;31:204–11.
Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab. 2015;17:849–58.
American Diabetes Association Workgroup on Hypoglycemia. Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care. 2005;28:1245–9.
Miyagawa J, Odawara M, Takamura T, Iwamoto N, Takita Y, Imaoka T. Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide is non-inferior to once-daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26-week randomized phase III study. Diabetes Obes Metab. 2015;2015(17):974–83.
Araki E, Inagaki N, Tanizawa Y, Oura T, Takeuchi M, Imaoka T. Efficacy and safety of once-weekly dulaglutide in combination with sulphonylurea and/or biguanide compared with once-daily insulin glargine in Japanese patients with type 2 diabetes: a randomized, open-label, phase III, non-inferiority study. Diabetes Obes Metab. 2015;17:994–1002.
Emoto M, Terauchi Y, Ozeki A, Oura T, Takeuchi M, Imaoka T. A 1-year safety study of dulaglutide in Japanese patients with type 2 diabetes on a single oral hypoglycemic agent: an open-label, nonrandomized, phase 3 trial. Endocr J. 2015;62:1101–14.
Skrivanek ZL, Gaydos BL, Chien JY, Geiger MJ, Heathman MA, Berry S, Anderson JH, Forst T, Milicevic Z, Berry D. Dose finding results in an adaptive, seamless, randomised trial of once weekly dulaglutide combined with metformin in type 2 diabetes (AWARD 5). Diabetes Obes Metab. 2014;16:748–56.
Wysham C, Blevins T, Arakaki R, Colon G, Garcia P, Atisso C, Kuhstoss D, Lakshmanan M. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care. 2014;37:2159–67.
Li L, Miao Z, Liu R, Yang M, Liu H, Yang G. Liraglutide prevents hypoadiponectinemia-induced insulin resistance and alterations of gene expression involved in glucose and lipid metabolism. Mol Med. 2011;17:1168–78.
Chelliah A, Burge MR. Hypoglycaemia in elderly patients with diabetes mellitus: causes and strategies for prevention. Drugs Aging. 2004;21:511–30.
Japan Diabetes Society. Committee on proper use of incretin drugs (GLP-1 receptor agonists and DPP-4 inhibitors), 2011 Available from http://www.fa.kyorin.co.jp/ jds/uploads/photos/797.pdf (in Japanese). Accessed 29 June 2017.
Boustani MA, Pittman I 4th, Yu M, Thieu VT, Varnado OJ, Juneja R. Similar efficacy and safety of once-weekly dulaglutide in patients with type 2 diabetes aged ≥ 65 and < 65 years. Diabetes Obes Metab. 2016;18:820–8.
Hamano K, Nishiyama H, Matsui A, Sato M, Takeuchi M. Efficacy and safety analyses across 4 subgroups combining low and high age and body mass index groups in Japanese phase 3 studies of dulaglutide 0.75 mg after 26 weeks of treatment. Endocr J. 2017;64:449–56.
Funding
This work was not supported by any Grant.
Author information
Authors and Affiliations
Contributions
TI contributed to the conception and design of the work. TI, TK, and MY contributed to the acquisition, analysis, and interpretation of data for the work. TI drafted the manuscript. TI, TK, and MY critically revised the manuscript for important intellectual content. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding author
Ethics declarations
Ethics approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revision.
Informed consent
Informed consent or substitute for it was obtained from all patients for being included in the study.
Conflict of interests
The authors declare that they have no competing interests.
About this article
Cite this article
Iwasaki, T., Kessoku, T., Higurashi, T. et al. Low body mass index and old age are useful in predicting the hemoglobin A1c-lowering effect of switching from sitagliptin to dulaglutide in Japanese patients with type 2 diabetes mellitus: a single-center, open-label, single-arm, pilot study. Diabetol Int 9, 189–195 (2018). https://doi.org/10.1007/s13340-018-0348-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-018-0348-0