Abstract
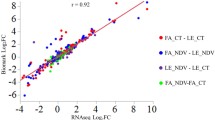
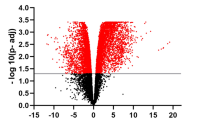
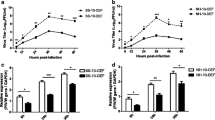
Newcastle disease (ND) affects a few hundred avian species including chicken and several species of domestic and wild birds. The clinical outcome of Newcastle disease virus (NDV) infection ranges from mild to severe fatal disease depending on the NDV pathotype and the host species involved. Japanese quails serve as natural reservoirs of NDV and play important role in NDV epidemiology. While infection of chicken with velogenic NDV results in severe often fatal illness, the same infection in Japanese quails results in inapparent infection. The molecular basis of this contrasting clinical outcomes of NDV infection is not yet clearly known. We compared global gene expression in spleen of chicken and Japanese quails infected with lentogenic and velogenic NDVs. We found contrasting regulation of key genes associated with NF-κB pathway and T-cell activation between chicken and Japanese quails. Our data suggests association of NDV resistance in Japanese quails to activation of NF-κB pathway and T cell proliferation.




Similar content being viewed by others
Availability of data and materials
Transcriptome data is available at GEO database accession number GSE98296.
References
Rehmani SF, Wajid A, Bibi T, Nazir B, Mukhtar N, Hussain A, Lone NA, Yaqub T, Afonso CL. Presence of virulent Newcastle disease virus in vaccinated chickens in farms in Pakistan. J Clin Microbiol. 2015;53(5):1715–8.
Mayo MA. A summary of taxonomic changes recently approved by ICTV. Arch Virol. 2002;147(8):1655–63.
Dai Y, Liu M, Cheng X, Shen X, Wei Y, Zhou S, Yu S, Ding C. Infectivity and pathogenicity of Newcastle disease virus strains of different avian origin and different virulence for mallard ducklings. Avian Dis. 2013;57(1):8–14.
Dortmans JC, Koch G, Rottier PJ, Peeters BP. Virulence of Newcastle disease virus: what is known so far? Vet Res. 2011;42:122.
Munir S, Sharma JM, Kapur V. Transcriptional response of avian cells to infection with Newcastle disease virus. Virus Res. 2005;107(1):103–8.
Lan D, Tang C, Li M, Yue H. Screening and identification of differentially expressed genes from chickens infected with Newcastle disease virus by suppression subtractive hybridization. Avian Pathol. 2010;39(3):151–9.
Balogh A, Bator J, Marko L, Nemeth M, Pap M, Setalo G Jr, Muller DN, Csatary LK, Szeberenyi J. Gene expression profiling in PC12 cells infected with an oncolytic Newcastle disease virus strain. Virus Res. 2014;185:10–22.
Glennon NB, Jabado O, Lo MK, Shaw ML. Transcriptome profiling of the virus-induced innate immune response in Pteropus vampyrus and its attenuation by Nipah Virus interferon antagonist functions. J Virol. 2015;89(15):7550–66.
Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57–63.
Liu WQ, Tian MX, Wang YP, Zhao Y, Zou NL, Zhao FF, Cao SJ, Wen XT, Liu P, Huang Y. The different expression of immune-related cytokine genes in response to velogenic and lentogenic Newcastle disease viruses infection in chicken peripheral blood. Mol Biol Rep. 2012;39(4):3611–8.
Japanese Quail (Coturnix coturnix japonica) as Newcastle Disease Virus Carrie
Ranaware PB, Mishra A, Vijayakumar P, Gandhale PN, Kumar H, Kulkarni DD, Raut AA. Genome wide host gene expression analysis in chicken lungs infected with Avian Influenza Viruses. PLoS ONE. 2016;11(4): e0153671.
Kuchipudi SV, Tellabati M, Sebastian S, Londt BZ, Jansen C, Vervelde L, Brookes SM, Brown IH, Dunham SP, Chang KC. Highly pathogenic avian influenza virus infection in chickens but not ducks is associated with elevated host immune and pro-inflammatory responses. Vet Res. 2014;45:118.
Kusumawidjaja G, Kayed H, Giese N, Bauer A, Erkan M, Giese T, Hoheise JD, Friess H, Kleeff J. Basic transcription factor 3 (BTF3) regulates transcription of tumor-associated genes in pancreatic cancer cells. Cancer Biol Ther. 2007;6(3):367–76.
Chhabra R, Kuchipudi SV, Chantrey J, Ganapathy K. Pathogenicity and tissue tropism of infectious bronchitis virus is associated with elevated apoptosis and innate immune responses. Virology. 2016;488:232–41.
Poenisch M, Metz P, Blankenburg H, Ruggieri A, Lee JY, Rupp D, Rebhan I, Diederich K, Kaderali L, Domingues FS, et al. Identification of HNRNPK as regulator of hepatitis C virus particle production. PLoS Pathog. 2015;11(1): e1004573.
Wang J, Basagoudanavar SH, Wang X, Hopewell E, Albrecht R, Garcia-Sastre A, Balachandran S, Beg AA. NF-kappa B RelA subunit is crucial for early IFN-beta expression and resistance to RNA virus replication. J Immunol. 2010;185(3):1720–9.
Li Y, Zhang P, Wang C, Han C, Meng J, Liu X, Xu S, Li N, Wang Q, Shi X, et al. Immune responsive gene 1 (IRG1) promotes endotoxin tolerance by increasing A20 expression in macrophages through reactive oxygen species. J Biol Chem. 2013;288(23):16225–34.
Tallam A, Perumal TM, Antony PM, Jager C, Fritz JV, Vallar L, Balling R, Del Sol A, Michelucci A. Gene Regulatory Network Inference of Immunoresponsive Gene 1 (IRG1) Identifies Interferon Regulatory Factor 1 (IRF1) as its transcriptional regulator in mammalian macrophages. PLoS ONE. 2016;11(2): e0149050.
Preusse M, Tantawy MA, Klawonn F, Schughart K, Pessler F. Infection- and procedure-dependent effects on pulmonary gene expression in the early phase of influenza A virus infection in mice. BMC Microbiol. 2013;13:293.
Ren K, Lv Y, Zhuo Y, Chen C, Shi H, Guo L, Yang G, Hou Y, Tan RX, Li E. Suppression of IRG-1 reduces inflammatory cell infiltration and lung injury in respiratory syncytial virus infection by reducing production of reactive oxygen species. J Virol. 2016;90(16):7313–22.
Lasoudris F, Cousin C, Prevost-Blondel A, Martin-Garcia N, Abd-Alsamad I, Ortonne N, Farcet JP, Castellano F, Molinier-Frenkel V. IL4I1: an inhibitor of the CD8(+) antitumor T-cell response in vivo. Eur J Immunol. 2011;41(6):1629–38.
Yue Y, Huang W, Liang J, Guo J, Ji J, Yao Y, Zheng M, Cai Z, Lu L, Wang J. IL4I1 is a novel regulator of M2 macrophage polarization that can inhibit T cell activation via L-Tryptophan and Arginine depletion and IL-10 production. PLoS ONE. 2015;10(11): e0142979.
Boulland ML, Marquet J, Molinier-Frenkel V, Moller P, Guiter C, Lasoudris F, Copie-Bergman C, Baia M, Gaulard P, Leroy K, et al. Human IL4I1 is a secreted L-phenylalanine oxidase expressed by mature dendritic cells that inhibits T-lymphocyte proliferation. Blood. 2007;110(1):220–7.
Salomonsen J, Sorensen MR, Marston DA, Rogers SL, Collen T, van Hateren A, Smith AL, Beal RK, Skjodt K, Kaufman J. Two CD1 genes map to the chicken MHC, indicating that CD1 genes are ancient and likely to have been present in the primordial MHC. Proc Natl Acad Sci U S A. 2005;102(24):8668–73.
Zhang L, Li P, Liu R, Zheng M, Sun Y, Wu D, Hu Y, Wen J, Zhao G. The identification of loci for immune traits in chickens using a genome-wide association study. PLoS ONE. 2015;10(3): e0117269.
Paschalidis N, Huggins A, Rowbotham NJ, Furmanski AL, Crompton T, Flower RJ, Perretti M, D’Acquisto F. Role of endogenous annexin-A1 in the regulation of thymocyte positive and negative selection. Cell Cycle. 2010;9(4):784–93.
Zhang Z, Huang L, Zhao W, Rigas B. Annexin 1 induced by anti-inflammatory drugs binds to NF-kappaB and inhibits its activation: anticancer effects in vitro and in vivo. Cancer Res. 2010;70(6):2379–88.
Acknowledgements
The authors thank the Indian Council of Agricultural Research, Government of India, New Delhi for funding this work through the Niche area of Excellence in Animal Biotechnology program and the Professor and Head, Department of Animal Biotechnology, Madras Veterinary College, The Professor and Head, Poultry Research Station, Madharavam and the Dean, Faculty Basic Sciences, Tamil Nadu Veterinary and Animal Sciences University (TANUVAS) for extending the required facilities.
Funding
The study was funded in part by DST-INSPIRE program of The Department of Science and Technology (awarded to MKPS), a UKIERI Trilateral Research in Partnership Award (ND/CONT/E/12–13/704) and start up research grants of the Department of Veterinary and Biomedical Sciences, the Pennsylvania State University (SVK).
Author information
Authors and Affiliations
Contributions
SVK and KK conceived the study. KK, VK and MKPS designed the experiments and MKPS carried out the experiments. RHN, MKPS and JD analyzed the data, MKPS, VK, KK, JD and SVK interpreted the data. MKPS, KK, RHN and SVK prepared the manuscript and all authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval and consent to participate
Animal infection study was approved by the Institutional Animal Ethics Committee (IAEC) of TANUVAS (Approval Number: 3028/DFBS/2014) and all experiments were performed according to institutional guidelines.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Panner Selvam, M.K., Kanagaraj, V., Kathaperumal, K. et al. Comparative transcriptome analysis of spleen of Newcastle Disease Virus (NDV) infected chicken and Japanese quail: a potential role of NF-κβ pathway activation in NDV resistance. VirusDis. 34, 402–409 (2023). https://doi.org/10.1007/s13337-023-00833-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-023-00833-y