Abstract
Several infections have emerged in humans, domestic animals, wildlife, and plant populations, causing a severe problem for humanity. Since the discovery of the Monkeypox virus (Mpox) in 1958 in Copenhagen, Denmark, it has resurfaced several times, producing severe infections in humans and resulting in a significant fatality rate. Mpox is an Orthopoxvirus of the Poxviridae family. This family contains various medically important viruses. The natural reservoir of Mpox is unknown yet. Mpox might be carried by African rodents and nonhuman primates (such as monkeys). The role of monkeys has been confirmed by its various outbreaks. The infection may be transferred from unidentified wild animals to monkeys, who can then spread it to humans by crossing species barriers. In close contact, human-to-human transmission is also possible. Mpox outbreaks have been documented regularly in Central and Western Africa, but recently in 2022, it has spread to over one hundred-six countries. There is no specific treatment for it, although the smallpox vaccine, antivirals, and vaccinia immune globulin help in the effective management of Mpox. In conclusion: Monkeypox poses a severe threat to public health due to the lack of specific vaccinations and effective antivirals. Surveillance studies in affected regions can assist in the early diagnosis of disease and help to control significant outbreaks. The present review provides information on epidemiology, clinical symptoms, risk factors, diagnosis, and preventive measures of Mpox.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The newly emerging and re-emerging viral infection is a significant public health concern. Infectious agents emerge due to imbalances in various ecological, environmental, or demographic factors. Deforestation, global warming, changing climate, high population density, unregulated development, poor sanitation, and vector adaptability put selection pressure on host–pathogen reservoirs, resulting in various viral emergence and re-emergence. Outbreaks of Crimean Congo hemorrhagic fever (CCHF), Ebola hemorrhagic fever (EHF), Lassa hemorrhagic fever, HIV-1, Marburg virus (MARV), SARS-CoV, MERS-CoV, Nipah virus (NiV) [49], Zika virus (ZIKV) [52], Rift Valley fever (RVF), Cat Que Virus [53] and COVID-19 are only a few among of several outbreaks in last few decades. Because of the unexpected recent Monkeypox virus (Mpox) epidemic, people worldwide should exercise care and be aware of the virus and its spread. Mpox was initially identified in research centre in Copenhagen, Denmark, in 1958 as an agent of a pox-like infection in monkeys [35]. The term 'Monkeypox' was coined for pox-like infectious outbreaks observed in the population of Cynomolgus monkeys (Macaca fascicularis) [32].
The first human case of Mpox infection was discovered in the Democratic Republic of Congo in 1970, shortly after Smallpox was eradicated. Undoubtedly, prior occurrences of Mpox were possible in Central Africa, but they were most likely misdiagnosed, due to the high prevalence of Smallpox. Human infection with Mpox has been frequently documented in several central and western African countries and sometimes outside Africa. Mpox is a large, double-stranded DNA virus that belongs to the Orthopoxvirus genus of the Poxviridae family. Skunkpox, Volepox, Raccoonpox, Cowpox, Variola, Camelpox, Ectromelia, Teterapox, Uasin Gishu disease, and Vaccinia viruses of this family other than Monkeypox also cause infection in humans [38]. Smallpox was a highly infectious disease of the Poxviridae family. It was responsible for the killing of up to 30% of people. Due to an efficient vaccination effort, the natural infection has been eradicated.
Monkeypox is a zoonotic viral infection caused by the Monkeypox virus carried by infected rodents and squirrels native to central and western Africa. Secondary transmission from person to person is also possible, but far less than Smallpox. The incubation period of Monkeypox virus is similar to Smallpox virus. The clinical symptoms of Monkeypox in human are comparable to Smallpox but less severe. Fever, headache, muscular pains and fatigue are among the prominent clinical symptoms of Monkeypox. It causes lymph node swelling (lymphadenopathy), which is absent or rare in the case of Smallpox. The symptoms of Monkeypox usually subside on their own after a few weeks, but Monkeypox can cause more severe symptoms and even death in newborns, infants, and those with underlying immune weaknesses. Mpoxs are divided into two genetic clades: the Congo Basin clade, which causes a case fatality rate of 2–10%, and the West African milder clade, which causes low mortality in Smallpox virus unvaccinated persons [7, 34]. Due to the severe disease caused by Mpox, it requires a quick, sensitive, precise, and cost-effective therapy. There is no confirmed Mpox-specific vaccination or antiviral for its effective treatment. The previous Mpox outbreaks were managed with smallpox vaccination, antivirals, and vaccinia immune globulin (VIG). Smallpox vaccination, antivirals, vaccinia immune globulin, and other symptomatic therapy effectively reduced the Mpox disease symptoms. The present review focuses on distribution, clinical symptoms, risk factors, diagnosis, and preventive measures of Monkeypox viruses.
The causative agent of Monkeypox
Poxviridae is one of the prominent viral families containing various medically important viruses like Variola (VARV), Cowpox (CPX), Vaccinia (VACV), and Monkeypox virus (Mpox). Mpox is an enveloped, double-stranded DNA virus that belongs to the genus Orthopoxvirus and the family Poxviridae. It has a sophisticated internal structure that comprises a double-stranded DNA genome (130–260 kb) and various types of enzymes. The detailed structure of the Mpox virus is given in Fig. 1
It is a brick-shaped virus with distinctive surface tubules and a 200–250 nm dumbbell-shaped core component. The Poxviridae family has two subfamilies (Chordopoxvirinae and Entomopoxvirinae). Orthopoxvirus, Molluscipoxvirus, Parapoxvirus, and Yatapoxvirus of Chordopoxvirinae can naturally infect humans. The Orthopox genus has 11 distinct species of viruses that are antigenically and physically similar. Skunkpox, Volepox, and Raccoonpox are three North American viruses, whereas Monkeypox, Cowpox, Variola, Camelpox, Ectromelia, Teterapox, Uasin Gishu disease, and Vaccinia are eight Eurasian-African viruses [38]. According to a computational study, the Monkeypox virus has a big genome with 196,858 base pairs that encode 190 open reading frames with 60 amino acid residues [3, 43]. Viruses with DNA genomes can replicate in the host cell's nucleus, while orthopoxviruses survive in the host cell's cytoplasm and replicate there. Its genome encoded all of the proteins required for viral replication, virion assembly, transcription, and translation (Fig. 2). Among the Orthopoxvirus, housekeeping genes are also found in the conserved area (central region). The Monkeypox virus-host interaction is mainly controlled by the genes located at the termini (ITRs, STRs) and Hairpin loops [4, 16, 46]. There is a difference between infectious Mpox virions isolated from infected cells in animal culture and the patient directly. Mpox virions isolated from animal culture do not contain an extra outer membrane, as do naturally released virions obtained from the patient.
Mpox is a zoonotic virus that infected rats and squirrels may transfer, turning central and western Africa into endemic regions. Mpox can affect a variety of mammals, but rodents are most likely to be the reservoir of the virus. There is the potential that primates may have reserves. Mpox has only been found in wild animals like Thomas's rope squirrel (Funisciurus anerythrus), Cercocebus arys, Pan troglodytes, Pouched rats, and African dormice. These animals shed high Mpox titers in oral, nasal, and rectal secretions, which might have contaminated the environment and spread to other animals [15]. Earl et al., [12] study also showed that the virus was growing in the noses of infected dormice five days after infection. Infected rats and monkeys can spread Mpox through skin or mucosal contact [15]. Animal studies might provide the most likely transmission channels to determine how long these animals can keep and shed the virus and simulate Mpox transmission in different species. Secondary transmission of Mpox from one person to another is conceivable, although considerably less often than Smallpox. It is transmitted by sexual activity, as seen recently [14]. The severity of Mpox can be from mild to life-threatening. Mpox has two genetic clades: a Congo basin clade related to 2–10% death in Smallpox unvaccinated persons and a West African clade linked to milder illness and exceptionally low mortality in Smallpox unvaccinated people [7, 34].
Epidemiology of Mpox
Monkeypox virus has a long history of prevalence in central and western Africa, although smallpox obscured it because of symptomatic similarities. After Smallpox was eradicated, Mpox instances came to light and became more prominent. Mpox is a zoonotic virus that may be transmitted from a reservoir animal to humans. In rare circumstances, Mpox has been transmitted from person to person. The rodents or squirrels living in the Sub-Saharan Africa rain forests act as potential reservoirs, amplifying Mpox. Humans and monkeys are accidental Mpox hosts [31]. Between 1970 and 1979, only 47 cases of Mpox were documented worldwide. Thirty-eight of them are from the Democratic Republic of Congo, with the rest being from Gabon, the Ivory Coast, Liberia, Nigeria, and Sierra Leone in western Africa. Most Mpox cases in the Democratic Republic of Congo have animal contact histories. Out of these 47 patients, seven (14.9%) died, while 40 (85.1%) recovered. Person-to-person transmissions amongst 7.5% of family members were also noted [5]. Due to the significant impact of Mpox on human health and wealth, World Health Organization (WHO) conducted surveillance from 1981 to 88 to check Mpox prevalence in the Democratic Republic of Congo (DRC). The Mpox surveillance in DRC revealed that over 70% of human cases had direct animal contact history. It was also observed that Mpox secondary transmission (person-to-person) occurs at a substantial rate of 30%. The Mpox monitoring revealed a critical role in animal transmission. Children (mean age 4.4 years) were more susceptible to Mpox infection. The smallpox vaccination or herd immunity against Smallpox can protect people from contracting Monkeypox because Smallpox and Monkeypox viruses are closely related. According to historical evidence from Africa, the smallpox vaccination is at least 85% effective in preventing Monkeypox. Smallpox vaccine recipients have a low prevalence of Mpox, indicating a low risk of an Mpox epidemic [22, 23].
Human Mpox cases decreased after 1986, with just 13 cases recorded from 1986 to 1992 and no patients reported from 1993 to 1995 [20, 42]. In the Democratic Republic of Congo's Kasai-Oriental province, an outbreak of Mpox was recorded in 1996–97. There were over 500 cases of Mpox, with a lower fatality rate (1–5%), but a significant incidence of man-to-man transmission was higher as compared to previous epidemics (78%). The Ministry of Health of the Democratic Republic of Congo conducted surveillance during 1998–2002, and more than 1200 cases of Mpox were found [30]. In 2003, the first human Monkeypox cases in the Western Hemisphere were recorded following an epidemic in the Midwest USA (Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin). It was caused by the importation of Monkeypox-infected West African rodents from Ghana. In total, 72 incidents of Mpox were noted based on clinical presentation. Out of these, 37 were further confirmed with laboratory testing. This epidemic raised awareness of the health risks associated with the worldwide commerce of exotic animals. Presently Mpox has its footprints outside of Africa. Numerous human cases of infection have been documented in Israel (2018), Singapore (2019), the United Kingdom (UK) (2018, 2021), and the United States of America (USA) (2003, 2021) [60]. Now recent outbreak of Mpox has occurred in 106 nations (2022), and WHO declared the Mpox outbreak as public health emergency of international concern (PHEIC) on 23 July 2022.
Recent outbreak of Monkeypox virus (Mpox) disease in various countries
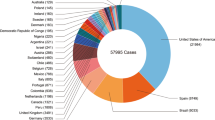
The Monkeypox virus has a long prevalent history in Central and Western Africa. Based on the prevalence of Mpox, a country may be termed as endemic or non-endemic. An epidemic is defined as a single incidence of Monkeypox in a non-endemic country. Mpox has expanded its range from endemic to non-endemic nations all of a sudden. Till September 21, 2022, One hundred and six countries have reported human cases of Mpox, and there will be more chance of more expansion of Mpox cases. Due to the increase in international travel and low awareness among the population, this may further rise. Furthermore, WHO expects more cases of Monkeypox to be discovered as surveillance expands in non-endemic countries. Epidemiological investigations are underway; as of September 21, 2022, sixty-one thousand and seven hundred fifty-three (61,753) confirmed Monkeypox cases, and twenty-three (23) deaths have been reported to WHO from 105 countries [61] (Table 1). Most Mpox cases have been reported through general care or reproductive health care. It is challenging to trace the probable cases of Mpox in cases without a travel history to an endemic region. According to a preliminary epidemiological study, Mpox is spread sexually among men who have sex with other men (MSM). The emergence of Monkeypox in several nations simultaneously shows that undetected transmission has been going on for some time, as well as recent amplifying events. In certain nations, however, the offender is given a travel warning. However, some countries issue traveler advisories to the passenger and carry surveillance on the traveler, people close to him before his hospitalization, and hospital health workers.
Clinical symptoms of Mpox
Mpox can enter the body through skin abrasions, the mucosa of the upper respiratory tract, or through ingestion. The Monkeypox virus incubation period ranges from 5 to 21 days. The commencement of the prodromal stage is marked by the appearance of early symptoms of Mpox, which are similar to those of other viral illnesses (e.g. fever, malaise, headache, weakness, etc.). Early investigations showed that Mpox was similar to Smallpox regarding clinical symptoms, morbidity, and mortality. Compared to the smallpox virus, Mpox has a modest transmission rate from person to person [5]. Smallpox and Mpox cannot be distinguished based on clinical symptoms, requiring the use of a specialized laboratory confirmation assay for their accurate diagnosis and subsequent treatments. The development of lymphadenopathy is one clinical feature that distinguishes Mpox from Smallpox. In Monkeypox, lymph node swelling can be widespread or restricted to a few locations (e.g. neck, and armpit). Cough, pharyngitis, chest tightness, nausea, diarrhea, myalgia, and back discomfort are some of Mpox infection's extra-cutaneous symptoms, (Fig. 3).
The rash typically appears one to three days after the fever has started. Cutaneous lesions can be flat or slightly elevated, filled with clear or yellowish fluid, crusted, dried up, and eventually fall off. A person's lesions can range from a few hundred to several thousand. The rash primarily affects the face, palms of the hands, and soles of the feet. They also appear on the lips, genitals, and eyes. The majority of patients recover without much difficulty. However, Secondary infections such as skin and soft tissue infections (SSTIs) (20%), pneumonitis (12%), ophthalmic (ocular) involvement (5%), and mild encephalitis (less than 1%) may cause issues for a small number of people [56]. Lesions in the mouth and on the body will appear after the prodrome. Before dropping off, lesions go through many phases. The lesions of an infectious person change from the beginning of the enanthem until the scab stage. The Enanthema phase follows the prodromal phase, which is marked by the development of red skin lesions or vesicular pustules that spread from the face to the entire body. These are hard, pea-size (10 mm in diameter) lesions that are painful. The status of smallpox vaccination, rather than sex or age, differentiates the occurrence and severity of clinical features of the Mpox [2]. Mpox can generally show milder infection and reduced morbidity and mortality in Smallpox pre-vaccinated cases. In recent years, 3–6% mortality was noted in endemic countries; most of the casualty happens in youngsters or those with underlying health problems. Also, the symptom of Mpox in animals are lethargy, coughing, running nose and eye, anorexia, fever, and a rash that looks like pimples or blisters [6].
Diagnosis of Mpox
Human Monkeypox is a reportable illness with a significant health impact; a quick, sensitive, specific, and cost-effective diagnosis and treatment should be necessary for effective management. Human Monkeypox can be accurately diagnosed via virus isolation, electron microscopy, conventional PCR, and Real-Time PCR. Immunohistochemical evidence of an orthopoxvirus in tissue can also be helpful for its diagnosis. Patient travel histories along with clinical symptoms are helpful for quick surveillance of Mpox. Virus isolation is considered the gold standard but time-consuming, and a higher bio-safety level is required. Mpox virus isolation can be done in the rhesus-monkey kidney, rabbit kidney, MRC-5, RD, B-SC- 40, Vero cells, and embryonated chicken eggs. Mpox produces CPE with rounded cells within 1–4 days, with significant cytoplasmic bridging and syncytium development. In embryonated chicken eggs, it causes tiny, opaque hemorrhagic pocks on the chorioallantoic membranes. In the case of emerging viruses, when there is no primer, probes, or specific cell line, then electron microscopy is very helpful for the initial diagnosis of viruses. An electron microscope is a very costly instrument, and highly specialized persons are required for viral diagnosis. Laboratory diagnosis of poxvirus infections through electron microscopy can rapidly exclude chickenpox (varicella) and a herpes virus, but it is challenging to differentiate similar shape viruses [9, 19]. The earliest evidence for an orthopoxvirus as a causative agent of the 2003 US epidemic came from a negative stain EM of cell culture supernatants [45]. Early management is better possible through rapid and accurate diagnosis. After the availability of primers and probes, conventional and/or Real-time PCR became dominant, effective, and reliable diagnosis assays in the modern era of science. Molecular diagnostic assays are comparatively better than other diagnostic methods for viral agents. Several molecular diagnostic techniques can help with the definitive diagnosis of Mpox infections. Two DNA-based diagnostic methods that can be considered more practical are PCR with restriction endonuclease digestion or hemagglutinin gene (HA) sequencing which can identify an Orthopoxvirus in a few hours. The molecular assay can provide further information about the sequencing of their genomes. Molecular diagnostic assays are comparatively more rapid, sensitive, specific, and economical. They can be performed at a lower bio-safety level than virus isolation. Serologic diagnostic assays work on the principle of antigen–antibody reaction and are less beneficial for the identity of Mpox infection. Due to their close antigenic relationships and cross-reactivity across members of the Poxviridae family.
Management of Mpox
Mpox has the potential to spread from one person to another. It poses a potential threat to become another pandemic [54]. Surveillance, accurate diagnosis, and patient management should be used to control Mpox, (Fig. 4) effectively. Several precautions may be taken to prevent the spread of Mpox infection. One should avoid touching animals that may harbor the Mpox or dead animals, especially in regions of the Mpox outbreak. Because Mpox is spread by direct contact, avoiding direct contact can help to minimize the risk of an epidemic. It is safer to stay away from anything that has come in contact with a sick animal, such as bedding where animals are housed in farms or laboratories. Similarly, interaction with Mpox-infected patients who have been isolated should be with extreme caution and with proper safety gear. There should be a physical barrier between Mpox patients and other patients and visitors. Immunocompromised patients, who are at a higher risk of Mpox or any other viral infection, should take extra precautions at all times and especially during the viral outbreak. To reduce the danger of transmitting Mpox infections, cleanliness and frequent hand-washing with soap and water or with an alcohol-based hand sanitizer should be followed after contact with infected animals or people. During the research and treatment of Mpox patients, one should wear personal protective equipment (PPE) and practice bio-safety precautions. Vaccines or antiviral drugs should be available to handle viral infections effectively, but unfortunately, no particular medicine or vaccinations are available. Hence Mpox patients can only be offered symptomatic therapy.
The smallpox vaccine provides substantial (85%) protection against Mpox infection. Persons at high risk of Mpox infection, such as those researching animal or human Monkeypox cases, health care professionals caring for infected patients, and laboratory workers handling potentially infectious materials, should undergo post-exposure smallpox vaccination. According to the CDC, immunization should be sought within four days after initial close contact with a confirmed Monkeypox case, and vaccination should be considered up to 14 days after exposure. Vaccinia immune globulin might be utilized as a preventive for people exposed but unable to be vaccinated due to low T-cell activity [18].
Several factors, including prior immunization history, baseline health, immune status, and comorbidity, influence the prognosis for Monkeypox. Hospitalization may be necessary for those with the hemorrhagic disease, confluent lesions, sepsis, encephalitis, or other disorders. Immunocompromised patients with leukemia, lymphoma, malignancy, solid organ transplantation, therapy with alkylating agents, antimetabolites, radiation, tumor necrosis factor inhibitors, high-dose corticosteroids, or a recipient of hematopoietic stem cell or organ transplant have the poor outcome of Mpox infection [44]. Similarly, pediatric populations, particularly patients younger than eight years of age, may have severe infections [23]. The atopic dermatitis patients and other active exfoliative skin diseases (e.g. eczema, burns, impetigo, varicella-zoster virus infection, herpes simplex virus infection, severe acne, severe diaper dermatitis with extensive areas of denuded skin, psoriasis) can experience severe disease complications with Mpox. Furthermore, Mpox infection can be risky in expecting or nursing women [8]; this would also be a case in persons with one or more complications, such as a secondary bacterial skin infection, gastroenteritis with severe nausea/vomiting, diarrhea, dehydration, bronchopneumonia or other comorbidities [41].
Medical countermeasures available for the treatment of Monkeypox
There is currently no medication explicitly approved for Mpox treatment. However, antivirals designed for people with Smallpox seem a viable option for Monkeypox. Adults and children can be treated for Smallpox using the antiviral drug Tecovirimat. Although there is no data on Tecovirimat's efficacy in treating human Monkeypox infections, research on various animal species shows promise that Tecovirimat is useful in treating diseases caused by orthopoxviruses. More detailed clinical studies on humans are warranted to establish that the medication will be safe and productive. In the absence of particular vaccinations or antiviral drugs, medicinal plant-based treatment may be a viable option. Compared to manufactured medicines, ethnomedicine is gaining popularity because of its safety and broad-spectrum action. Medicinal herbs are already effective in treating viral diseases such as Herpes Simplex Viruses, Dengue Fever, Chikungunya, etc. [24,25,26,27,28,29, 50, 51]. Various bioinformatics tools assist in more focused research and help narrow down more precise vaccinations or antivirals [21].
Vaccinia immune globulin intravenous (VIGIV)
VIGIV can treat post-vaccination problems such as eczema vaccinatum, progressive vaccinia, severe vaccinia, vaccinia infections in people with skin disorders, and aberrant infections by the vaccinia virus. So far, no data are available on the efficacy of VIG in treating Monkeypox virus infection. A person with a severe Monkeypox infection may benefit from receiving VIG therapy, although there is no evidence to support this claim. After pre-clinical studies on efficacy and safety, medical professionals could be allowed to use this for an emergency, at least in limited cases, under strict clinical trial protocols with due permission. As smallpox vaccination after exposure to the Monkeypox virus is prohibited, VIG can be investigated for preventive use in exposed individuals with severe impairment in T-cell function.
Antiviral drugs known as Cidofovir are frequently prescribed to people with HIV or CMV infection. Cidofovir can be administered to Mpox-infected individuals once detailed in vitro, and animal studies have demonstrated the efficacy of Cidofovir against orthopoxviruses and minimal toxicity to the host. Although it is unclear if Cidofovir therapy will benefit someone with a severe Mpox infection, it is worth studying that under strict ethical and clinical trial guidelines. Brincidofovir is another antiviral considered safer than Cidofovir in adults, pediatric, and newborns treated for Smallpox, this can also be tried for treatment for Mpox in vitro, and animal studies have established that it is effective against orthopoxviruses.
Prevention and control
The primary preventive measure for Monkeypox viral infection is to educate people about the risk factors and ways to reduce viral exposure. Monkeypox can spread from one person to another through close physical contact, including sexual activities. Some evidence suggests that Monkeypox can also spread through sexual transmission routes (e.g. through semen or vaginal fluids). Direct skin-to-skin contact with lesions during sexual activities can be one of the dominant causes of Mpox spread [21]. Health workers, relatives, and household members have a higher risk of Monkeypox infection as they are in close contact with patients during caregiving and treatment [42]. Handling and caring for infected or suspected persons should be done with extra precautions. If possible, a smallpox-vaccinated person should take care of the Mpox patient. As Mpox is a zoonotic virus, the infection can also spread from animal to human, avoiding contact with animals and their products and housing. A non-vegetarian should follow extra safety precautions regarding food consumption and handling. Suspected and Mpox-infected or contact animals should be isolated and quarantined promptly [42]. There should be rapid, sensitive, specific, and cost-effective diagnostic approaches for early and accurate diagnosis of Mpox. For effective management, scientists should follow new strategies like therapeutic agents, and particular vaccines, and target specific antivirals. Continuous surveillance of Mpox in humans and animals in the endemic region can act as a milestone and predict the outbreak [42].
Future perspective
The newly emerging and re-emerging viral infection is a significant public health concern of the current times. Crimean Congo hemorrhagic fever (CCHF), Ebola hemorrhagic fever (EHF), Lassa hemorrhagic fever, HIV-1, Marburg virus (MARV), SARS-CoV, MERS-CoV, Nipah virus (NiV), Zika virus (ZIKV), Rift Valley fever (RVF), Cat Que Virus infections, and COVID-19 disease are only a few of them. As an emerging virus, the Monkeypox virus has spread from Africa to other regions of the World, including India, and hence is a growing concern. It can cause infection in humans, domestic and wild animals, ranging from moderate to life-threatening consequences. There are limited diagnostic assays for the Mpox. Virus isolation, electron microscopy, traditional PCR, and Real-Time PCR can all be used to diagnose it reliably. There is no specific treatment for Mpox infection.
Without specialized vaccinations or antiviral drugs, medicinal plant-based treatment offers a viable option. Ethnomedicine is gaining popularity because of its safer and more broad-spectrum action. Medicinal herbs can aid in treating Monkeypox and other viruses by providing a rapid, sensitive, specific, and cost-effective therapy. Medicinal herbs are the most abundant sources of antibacterial compounds. Plants are a key way to find new medical compounds that can be used to make new drugs. Plants' secondary metabolites are also a source of medicines. Alkaloids, flavonoids, terpenoids, tannins, coumarins, quinones, carotenoids, and steroids are good sources of therapeutic agents. Each year, scientists take out several new secondary metabolites from plants. These provide a source of potential treatments for cancer, antimicrobial analgesic, etc. properties. Some of them can be highly used for the treatment of viral infections. Many medicinal herbs are already utilized to treat Herpes Simplex Viruses, Dengue Fever, Influenza, Chikungunya, etc. [24,25,26,27,28,29, 50, 51]. Many studies demonstrate that various combinations of ethanolic, methanolic, and aquatic plant extracts are employed to target the various viral replication pathways. In the situation of the reappearance of Monkeypox viruses, herbal remedies play a crucial role. Research reveals that Monkeypox and its associated viruses are not only effectively treated with plant-based therapeutics but also they are less toxic and have fewer side effects [1, 10, 17, 36, 37, 48].
Mpox is a well-known virus. It can potentially be employed as a bioterrorism agent and is hazardous. As a result, we should have a variety of antiviral drugs and an effective Mpox vaccine. There is an urgent need to establish sophisticated diagnostic tools and therapeutic agents for Mpox. Focus research efforts are warranted to develop in vitro and in silico technologies for designing therapeutic interventions with minimal side effects. Like in other viral infections, Mpox could be controlled by stringent measures against its spread, targeted antiviral medication and efficient vaccinations at war footing levels.
Conclusion
Mpox is an emerging disease that can cause mild to severe illness in humans across the World. Mpox and the smallpox virus belong to the same family. It has the potential to be used as a bioterrorism agent. Mpox outbreaks have been regularly documented in Central and Western Africa, but during the year 2022, it has spread to over 105 countries. There is also a chance of its spread at a larger scale in the future, which is a global concern. It is hence essential that nations should exchange information about emerging diseases like Mpox through the WHO. Monkeypox can have serious health consequences; a rapid, sensitive, specific, and cost-effective diagnosis is required for appropriate management. Human and animal surveillance should be done continuously to predict possible Mpox outbreaks. Except for the smallpox vaccination, which provides some protection, there is no particular therapy for Mpox infection. Medicinal plants are a rich source of secondary metabolites that may help offer natural and safe treatments without specific vaccines or antiviral medications for Mpox.
Data availability
All data relevant to the study is included in the article.
References
Abookleesh FL, Al-Anzi BS, Ullah A. Potential antiviral action of alkaloids. Molecules. 2022;27(3):903.
Alakunle E, Moens U, Nchinda G, et al. Monkeypox virus in Nigeria: infection biology, epidemiology, and evolution. Viruses. 2020;12(11):1257. https://doi.org/10.3390/v12111257.
Alkhalil A, Hammamieh R, Hardick J, et al. Gene expression profiling of Monkeypox virus-infected cells reveals novel interfaces for host-virus interactions. Virol J. 2010;7(1):1–9. https://doi.org/10.1186/1743-422X-7-173.
Barrett JW, McFadden G. Origin and evolution of poxviruses. Origin and evolution of viruses. Academic Press; 2008. p. 431–46.
Breman JG, Steniowski MV, et al. Human Monkeypox, 1970–1979. Bull World Health Organ. 1980;58(2):182.
Centers for disease control and prevention. Mpox. Pets in the Home. https://www.cdc.gov/poxvirus/Mpox/prevention/pets-in-homes.html (2022). Accessed 8 Dec 2022.
Chen N, Li G, Liszewski MK, et al. Virulence differences between Monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340(1):46–63. https://doi.org/10.1016/j.virol.2005.05.030.
Cono J, Cragan JD, Jamieson DJ, et al. Prophylaxis and treatment of pregnant women for emerging infections and bioterrorism emergencies. J Infect Dis. 2006;12(11):1631.
Curry A, Appleton H, Dowsett B. Application of transmission electron microscopy to the clinical study of viral and bacterial infections: present and future. Micron. 2006;37(2):91–106. https://doi.org/10.1016/j.micron.2005.10.001.
Dhawan BN. Anti-viral activity of Indian plants. Proc Natl Acad Sci India Sect B J Biol Sci. 2012;82:209–24.
Durski KN, McCollum AM, Nakazawa Y, et al. Emergence of Monkeypox—west and central Africa, 1970–2017. Morb Mortal Wkly Rep. 2018;67(10):306.
Earl PL, Americo JL, Cotter CA, Moss B. Comparative live bioluminescence imaging of Monkeypox virus dissemination in a wild-derived inbred mouse (Mus musculus castaneus) and outbred African dormouse (Graphiurus kelleni). Virology. 2015;475:150–8.
Erez N, Achdout H, Milrot E, et al. Diagnosis of imported Monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980.
European Centre for Disease Prevention and Control. Monkeypox cases reported in UK and Portugal. https://www.ecdc.europa.eu/en/news-events/monkeypox-cases-reported-uk-and-portugal (2022). Accessed 27 May 2022.
Falendysz EA, Lopera JG, Rocke TE, Osorio JE. Monkeypox Virus in animals: current knowledge of viral transmission and pathogenesis in wild animal reservoirs and captive animal models. Viruses. 2023;15(4):905.
Fenner F. The orthopoxviruses. Elsevier; 2012 December 2
Filippova EI. Antiviral activity of Lady’s mantle (Alchemilla vulgaris L.) extracts against orthopoxviruses. Bull Exp Biol Med. 2017;163(3):374–7.
Di Giulio DB, Eckburg PB. Human Monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4(1):15–25. https://doi.org/10.1016/S1473-3099(03)00856-9.
Hazelton PR, Gelderblom HR. Electron microscopy for rapid diagnosis of emerging infectious agents. Emerg Infect Dis. 2003;9(3):294.
Heymann DL, Szczeniowski M, Esteves K. Re-emergence of Monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54(3):693–702. https://doi.org/10.1093/oxfordjournals.bmb.a011720.
Jakhar R, Kaushik S, Gakhar SK. 3CL hydrolase-based multiepitope peptide vaccine against SARS-CoV-2 using immunoinformatics. J Med Virol. 2020;92(10):2114–23. https://doi.org/10.1002/jmv.25993.
Jezek Z, Arita I, Mutombo M, et al. Four generations of probable person-to-person transmission of human Monkeypox. Am J Epidemiol. 1986;123(6):1004–12. https://doi.org/10.1093/oxfordjournals.aje.a114328.
Ježek Z, Szczeniowski M, Paluku KM, et al. Human Monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156(2):293–8. https://doi.org/10.1093/infdis/156.2.293.
Kaushik S, Dar L, Kaushik S, et al. Identification and characterization of new potent inhibitors of dengue virus NS5 proteinase from Andrographis paniculata supercritical extracts on in animal cell culture and in silico approaches. J. Ethnopharmacol. 2021;267:113541. https://doi.org/10.1016/j.jep.2020.113541
Kaushik S, Dar L, Kaushik S, et al. Anti-dengue activity of supercritical extract and isolated oleanolic acid of Leucas cephalotes using in vitro and in silico approach. BMC Complement Med Ther. 2021;21(1):1–15. https://doi.org/10.1186/s12906-021-03402-2.
Kaushik S, Jangra G, Kundu V, et al. Antiviral activity of Zingiber officinale (Ginger) ingredients against the Chikungunya virus. VirusDisease. 2020;31(3):270–6. https://doi.org/10.1007/s13337-020-00584-0.
Kaushik S, Kaushik S, Kumar R, et al. In-vitro and in silico activity of Cyamopsis tetragonoloba (Gaur) L. supercritical extract against the dengue-2 virus. VirusDisease. 2020;31(4):470–8. https://doi.org/10.1007/s13337-020-00624-9.
Kaushik S, Kaushik S, Sharma V, et al. Antiviral and therapeutic uses of medicinal plants and their derivatives against dengue viruses. Phcogrev Rev. 2018;12(24):177–85. https://doi.org/10.4103/phrev.phrev_2_18.
Kaushik S, Sharma V, Chhikara S, et al. Anti-chikungunya activity of green synthesized silver nanoparticles using Carica papaya leaves in animal cell culture model. Asian J Pharm Clin Res. 2019;12(6):170–4. https://doi.org/10.22159/ajpcr.2019.v12i6.32179.
Kebela B. Le profil epidemiologique de Monkeypox en RDC 1998–2002. Bull Epidemiol Republ Democr Congo. 2004;29:2–6.
Khodakevich L, Ježek Z, Messinger D. Monkeypox virus: ecology and public health significance. Bull World Health Organ. 1988;66(6):747.
Ladnyj ID, Ziegler P, Kima E. A human infection caused by Monkeypox virus in Basankusu territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46(5):593.
Li Y, Olson VA, Laue T, et al. Detection of Monkeypox virus with real-time PCR assays. J Clin Virol. 2006;36(3):194–203. https://doi.org/10.1016/j.jcv.2006.03.012.
Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: Monkeypox viruses. J Gen Virol. 2005;86(10):2661–72. https://doi.org/10.1099/vir.0.81215-0.
Magnus PV, Andersen EK, Petersen KB, et al. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959;46(2):156–76. https://doi.org/10.1111/j.1699-0463.1959.tb00328.x.
Malabadi RB, Kolkar P, Chalannavar K. Human Monkeypox detected first time In India: role of traditional herbal treatment. Int J Sci res rev. 2022;4(12):3686–91.
Mazurkova NA, Protsenko MA, Filippova EI, Kukushkina TA, Vysochina GI, Lobanova IE, Mazurkov OY, Shishkina LN, Agafonov AP. Investigation of the antiviral activity of experimental samples obtained from the grass and roots of Alchemilla vulgaris L. against vaccinia virus and ectromelia virus. Drug Dev Regist. 2019;8(4):9–15.
Meyer H, Damon IK, Esposito JJ. Orthopoxvirus diagnostics. Methods Mol Biol. 2004;269:119–34. https://doi.org/10.1385/1-59259-789-0:119.
Newsweek. Monkeypox Outbreak Explosion as 1300 Suspected Cases Reported, 58 Deaths. https://www.newsweek.com/monkeypox-cases-democratic-republic-congo-europe-1708527 (2022). Accessed 12 Aug 2022.
Nguyen PY, Ajisegiri WS, Costantino V, et al. Reemergence of human Monkeypox and declining population immunity in the context of urbanization, Nigeria, 2017–2020. Emerg Infect Dis. 2021;27(4):1007.
Ogoina D, Iroezindu M, James HI, et al. Clinical course and outcome of human Monkeypox in Nigeria. Clin Infect Dis. 2020;71(8):e210–4. https://doi.org/10.1093/cid/ciaa143.
Parker S, Buller RM. A review of experimental and natural infections of animals with Monkeypox virus between 1958 and 2012. Future Virol. 2013;8(2):129–57. https://doi.org/10.2217/fvl.12.130.
Parker S, Nuara A, Buller RML, et al. Human Monkeypox: an emerging zoonotic disease. Future Microbiol. 2007;2(1):17–34. https://doi.org/10.2217/17460913.2.1.17.
Petersen BW, Harms TJ, Reynolds MG, et al. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupational exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. Morb Mortal Wkly Rep. 2016;65(10):257–62.
Reed KD, Melski JW, Graham MB, et al. The detection of Monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342–50. https://doi.org/10.1056/NEJMoa032299.
Remichkova M. Poxviruses: smallpox vaccine, its complications and chemotherapy. Virus Adapt Treat. 2010;2:41–6. https://doi.org/10.2147/VAAT.S8563.
Sadeuh-Mba SA, Yonga MG, Els M, et al. Monkeypox virus phylogenetic similarities between a human case detected in Cameroon in 2018 and the 2017–2018 outbreak in Nigeria. Infect Genet Evol. 2019;69:8–11. https://doi.org/10.1016/j.meegid.2019.01.006.
Sanna G, Madeddu S, Serreli G, Nguyen HT, Le NT, Usai D, Carta A, Cappuccinelli P, Zanetti S, Donadu MG. Antiviral effect of Hornstedtia bella Škorničk essential oil from the whole plant against vaccinia virus (VV). J Nat Prod. 2021;35(24):5674–80.
Sharma V, Kaushik S, Kumar R, et al. Emerging trends of Nipah virus: a review. Rev Med Virol. 2019;29(1):e2010. https://doi.org/10.1002/rmv.2010.
Sharma V, Kaushik S, Pandit P, et al. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl Microbiol Biotechnol. 2019;103(2):881–91. https://doi.org/10.1007/s00253-018-9488-1.
Sharma Y, Kawatra A, Sharma V, et al. In-vitro and in-silico evaluation of the anti-chikungunya potential of Psidium guajava leaf extract and their synthesized silver nanoparticles. VirusDis. 2021;32(2):260–5. https://doi.org/10.1007/s13337-021-00685-4.
Sharma V, Sharma M, Dhull D, et al. Zika virus: an emerging challenge to public health worldwide. Can J Microbiol. 2020;66(2):87–98. https://doi.org/10.1139/cjm-2019-0331.
Soniya K, Yadav S, Boora S, et al. The Cat Que virus: a resurfacing orthobunyavirus could lead to epidemics. VirusDisease. 2021;32(4):635–41. https://doi.org/10.1007/s13337-021-00745-9.
Taylor L. Monkeypox: WHO declares a public health emergency of international concern. Br Med J. 2022;378:o1874. https://doi.org/10.1136/bmj.o1874.
Vaughan A, Aarons E, Astbury J, et al. Human-to-human transmission of Monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26(4):782.
Weinstein RA, Nalca A, Rimoin AW, et al. Reemergence of Monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005;41(12):1765–71. https://doi.org/10.1086/498155.
World Health Organization. Monkeypox—Cameroon. https://www.who.int/emergencies/disease-outbreak-news/item/05-june-2018-monkeypox-cameroon-en (2018). Accessed 10 Aug 2022.
World Health Organization. Weekly bulletin on outbreak and other emergencies: week 26: 23–29 June 2018. https://apps.who.int/iris/handle/10665/272981 (2018). Accessed 30 May 2022.
World Health Organization. Monkeypox—Singapore. https://www.who.int/emergencies/disease-outbreak-news/item/16-may-2019-monkeypox-singapore-en (2019). Accessed 12 Aug 2022.
World Health Organization. Monkeypox. https://www.who.int/news-room/fact-sheets/detail/monkeypox (2022). Accessed 25 May 2022.
World Health Organization. Multi-country outbreak of Monkeypox, External situation report #6–21 September 2022. https://www.who.int/publications/m/item/multi-country-outbreak-of-monkeypox--external-situation-report--6---21-september-2022 (2022). Accessed 01 Sep 2022.
Funding
This research did not receive any specific grant from public, commercial, or not-for-profit funding agencies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boora, S., Yadav, S., Soniya, K. et al. Monkeypox virus is nature's wake-up call: a bird’s-eye view. VirusDis. 34, 191–203 (2023). https://doi.org/10.1007/s13337-023-00826-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-023-00826-x