Abstract
Background and Objective
Vancomycin is the drug of choice in the treatment of MRSA infections. In a published vancomycin population pharmacokinetic study on neonates in Singapore healthcare institutions, it was found that vancomycin clearance was predicted by weight, postmenstrual age, and serum creatinine. The aim of this study was to externally validate the vancomycin population pharmacokinetic model to develop a new dosage regimen in neonates, and to compare this regimen with the existing institutional and NeoFax® dosage regimens.
Methods
A retrospective chart review of neonates who received vancomycin therapy and therapeutic drug monitoring was conducted. The median prediction error percentage was calculated to assess bias, while the median absolute prediction error percentage and the root mean squared error percentage were calculated to assess precision. The new dosage regimen was developed using Monte Carlo simulation.
Results
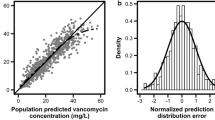
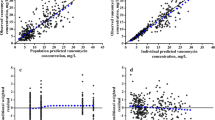
A total of 20 neonates were included in the external validation dataset. Eighteen of them were premature, with a median gestational age of 27.7 (25.9–31.5) weeks and postmenstrual age of 30.5 (27.3–34.3) weeks at the point of vancomycin initiation. No apparent systematic bias was found in the predictions of the model. The external validation performed in the current study found the model to be generally unbiased. Our new vancomycin dosage regimen was able to achieve target trough concentrations and area under the curve (AUC24) at a greater proportion as compared to existing institutional and NeoFax® dosage regimens.
Conclusion
The pharmacokinetic model built in the previous study can be used to conduct reliable population simulations of our Asian neonatal population in Singapore. The new dosage regimen was able to achieve target trough concentrations and AUC24 better than existing institutional and NeoFax® dosage regimens.




Similar content being viewed by others
References
Shah BA, Padbury JF. Neonatal sepsis: an old problem with new insights. Virulence. 2014;2014:170–8.
Schelonka RL, Infante AJ. Neonatal immunology. Semin Perinatol. 1998;22(1):2–14.
Boghossian NS, Page GP, Bell EF, Stoll BJ, Murray JC, Cotten CM, Shankaran S, Walsh MC, Laptook AR, Newman NS, Hale EC, McDonald SA, Das A, Higgins RD. Late-onset sepsis in very low birth weight infants from singleton and multiple-gestation births. J Pediatr. 2013;162:1120–4.
Shane AL, Hansen NI, Stoll BJ, Bell EF, Sanchez PJ, Shankaran S, Laptook AR, Das A, Walsh MC, Hale EC, Newman NS, Schrag SJ, Higgins RD. Methicillin-resistant and susceptible Staphylococcus aureus bacteremia and meningitis in preterm infants. Pediatrics. 2012;129:e914–22.
Nelson MU, Gallagher PG. Methicillin-resistant Staphylococcus aureus in the neonatal intensive care unit. Semin Perinatol. 2012;36:424–30.
Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91.
Jacqz-Aigrain E, Zhao W, Sharland M, van den Anker JN. Use of antibacterial agents in the neonate: 50 years of experience with vancomycin administration. Semin Fetal Neonatal Med. 2013;18:28–34.
Moellering RC Jr. Vancomycin: a 50-year reassessment. Clin Infect Dis. 2006;42(1):S3–4.
Pansieri C, Bonati M, Choonara I, Jacqz-Aigrain E. Neonatal drug trials: impact of EU and US paediatric regulations. Arch Dis Child Fetal Neonatal Ed. 2014;99:F438.
Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health-Syst Pharm. 2020;77:835–64.
Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–42.
Alvarez R, Lopez Cortes LE, Molina J, Cisneros JM, Pachon J. Optimizing the clinical use of vancomycin. Antimicrob Agents Chemother. 2016;60:2601–9.
Tseng SH, Lim CP, Chen Q, Tang CC, Kong ST, Ho PC. Evaluating the relationship between vancomycin trough concentration and 24-hour area under the concentration-time curve in neonates. Antimicrob Agents Chemother. 2018;62(4):e01647-e1717.
Liu YX, Wen H, Niu WJ, Li JJ, Li ZL, Jiao Z. External evaluation of vancomycin population pharmacokinetic models at two clinical centers. Front Pharmacol. 2021;12: 623907.
Koks CHW, Crommentuyn KML, Hoetelmans RMW, Mathôt RAA, Beijnen JH. Can fluconazole concentrations in saliva be used for therapeutic drug monitoring? Ther Drug Monit. 2001;23:4.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:2.
Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed. 2008;90(2):154–66.
Ryu S, Jung WJ, Jiao Z, Chae JW, Yun HY. External evaluation of the predictive performance of seven population pharmacokinetic models for phenobarbital in neonates. Br J Clin Pharmacol. 2021;87(10):3878–89.
Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59.
Truven Health Analytics, Inc. NeoFax® [Internet]. 2021. http://neofax.micromedexsolutions.com/neofax/neofax.php. Accessed 13 Dec 2021.
Le J, Ny P, Capparelli E, Lane J, Ngu B, Muus R, Romanowski G, Vo T, Bradley J. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4:e109–16.
Zasowski EJ, Murray KP, Trinh TD, Finch NA, Pogue JM, Mynatt RP, Rybak MJ. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob Agents Chemother. 2017;62:1.
Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B, Girard P, Laffont CM, Mentre F. Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet. 2007;46:221–34.
Sinkeler FS, de Haan TR, Hodiamont CJ, Bijleveld YA, Pajkrt D, Mathôt RAA. Inadequate vancomycin therapy in term and preterm neonates: a retrospective analysis of trough serum concentrations in relation to minimal inhibitory concentrations. BMC Pediatr. 2014;14:193.
Leroux S, Jacqz-Aigrain E, Biran V, Lopez E, Madeleneau D, Wallon C. Clinical utility and safety of a model-based patient–tailored dose of vancomycin in neonates. Antimicrob Agents Chemother. 2016;60:2039–42.
Tauzin M, Cohen R, Durrmeyer X, Dassieu G, Barre J, Caeymaex L. Continuous-infusion vancomycin in neonates: assessment of a dosing regimen and therapeutic proposal. Front Pediatr. 2019;7:188.
Demirel B, Imamoglu E, Gursoy T, Demirel U, Topcuoglu S, Karatekin G, Demirel U. Comparison of intermittent versus continuous vancomycin infusion for the treatment of late-onset sepsis in preterm infants. J Neonatal Perinat Med. 2015;8:149–55.
Gwee A, Cranswick N, McMullan B, Perkins E, Bolisetty S, Gardiner K, Daley A, Ward M, Chiletti R, Donath S, et al. Continuous versus intermittent vancomycin infusions in infants: a randomized controlled trial. Pediatrics. 2019;143: e20182179.
Frymoyer A, Stockmann C, Hersh AL, Goswami S, Keizer RJ. Individualized empiric vancomycin dosing in neonates using a model-based approach. J Pediatric Infect Dis Soc. 2019;8(2):97–104.
Lee SM, Yang S, Kang S, Chang MJ. Population pharmacokinetics and dose optimization of vancomycin in neonates. Sci Rep. 2021;11:6168.
Weintraub AS, Carey A, Connors J, Blanco V, Green RS. Relationship of maternal creatinine to first neonatal creatinine in infants < 30 weeks gestation. J Perinatol. 2015;35:401–40.
Acknowledgements
We thank Dr Sing Teang Kong for her valuable input on the external validation of our vancomycin pharmacokinetic model.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Ethics Approval
This retrospective chart review study was in line with the principles of the Declaration of Helsinki. Approval was granted by the NHG Domain Specific Review Board.
Consent to Participate
A waiver of informed consent was granted by the NHG Domain Specific Review Board.
Consent to Publish
Not applicable.
Author Contributions
All authors contributed to the study conception and design. Material preparation and data collection and analysis were performed by Chuan Poh Lim and Sheng Hsuan Tseng. The first draft of the manuscript was written by Chuan Poh Lim and Sheng Hsuan Tseng. All authors commented on previous versions of the manuscript and read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Lim, C.P., Tseng, S.H., Neoh, C.C.C. et al. External Validation of a Vancomycin Population Pharmacokinetic Model and Developing a New Dosage Regimen in Neonates. Eur J Drug Metab Pharmacokinet 47, 687–697 (2022). https://doi.org/10.1007/s13318-022-00781-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00781-w