Abstract
Background and Objectives
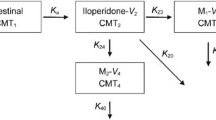
Risperidone is a derivative of benzisoxazole and is widely used for schizophrenia and other psychiatric illnesses in both adults and children. Previous studies have confirmed that it is a highly variable drug (within-subject variability ≥ 30%). To reduce the large sample size required for bioequivalence researches on highly variable drugs, a role for genotyping in the design of the bioequivalence study was employed.
Methods
A randomized, open-label, two-period crossover study was adopted: 20 subjects with specific genotypes carrying cytochrome P450 (CYP) 2D6*10 were randomized to two groups to receive a single oral dose of trial formulation or reference formulation with a 2-week washout period. Blood concentrations of risperidone (parent drug) and 9-hydroxy risperidone (active metabolite) were measured by high-performance liquid chromatography–tandem mass spectrometry.
Results
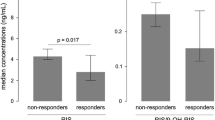
Eighteen out of the 20 subjects completed the study (two did not finish the test in the second period). The pharmacokinetic parameters of AUClast, AUC∞ and Cmax for the 18 subjects after a single oral dose of the trial or reference preparation were 216.1 ± 88.7 and 220.5 ± 96.8 ng·h/mL; 221.6 ± 93.1 and 226.4 ± 103.5 ng·h/mL; 36.7 ± 10.3 and 36.0 ± 10.2 ng/mL, respectively. The CVw of risperidone in natural logarithm-transformed Cmax was 22.4 and 25.38% for 9-hydroxy risperidone.
Conclusions
The test formulation met the Food and Drug Administration guidelines and regulation criteria for bioequivalence. By controlling the genotype, it could actually help reduce the CVw, which may be a feasible method to decrease the sample size for the bioequivalence study of highly variable drugs.



Similar content being viewed by others
References
He H, Richardson JS. A pharmacological, pharmacokinetic and clinical overview of risperidone, a new antipsychotic that blocks serotonin 5-HT2 and dopamine D2 receptors. Int Clin Psychopharmacol. 1995;10:19–30.
Leysen JE, Gommeren W, Eens A, de Chaffoy de Courcelles D, Stoof JC, Janssen PA. Biochemical profile of risperidone, a new antipsychotic. J Pharmacol Exp Ther. 1998;247:661–70.
Chouinard G, Arnott W. Clinical review of risperidone. Can J Psychiatr. 1993;38(Suppl 3):S89–95.
Mannens G, Huang ML, Meuldermans W, Hendrickx J, Woestenborghs R, Heykants J. Absorption, metabolism and excretion of risperidone in humans. Drug Metab Dispos. 1993;21(6):1134–41.
Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharm J. 2005;5(1):6–13.
Heykants J, Huang ML, Mannens G, Meuldermans W, Snoeck E, Van Beijsterveldt L, Van Peer A, Woestenborghs R. The pharmacokinetics of risperidone in humans: a summary. J Clin Psychiatr. 1994;55(Suppl):13–7.
Huang ML, van Peer A, Woestenborghs R, De Coster R, Heykants J, Jansen AA, Zylicz Z, Visscher HW, Jonkman JH. Pharmacokinetics of the novel antipsychotic agent risperidone and the prolactin response in healthy subjects. Clin Pharmacol Ther. 1993;54:257–68.
Ereshefsky L, Lacombe S. Pharmacological profile of risperidone. Can J Psychiatr. 1993;38(Suppl 3):S80–8.
Cho Hea-Young, Lee Young-Bok. Pharmacokinetics and bioequivalence evaluation of risperidone in healthy male subjects with different CYP2D6 genotypes. Arch Pharm Res. 2006;29(6):525–33.
González-Vacarezza N, Abad-Santos F, Carcas-Sansuan A, Dorado P, Peñas-Lledó E, Estévez Carrizo F, Llerena A. Use of pharmacogenetics in bioequivalence studies to reduce sample size: an example with mirtazapine and CYP2D6. Pharm J. 2013;13(5):452–5.
Cabaleiro T, Ochoa D, Román M, Moreno I, López-Rodríguez R, Novalbos J, Abad-Santos F. Polymorphisms in CYP2D6 have a greater effect on variability of risperidone pharmacokinetics than gender. Basic Clin Pharmacol Toxicol. 2015;116(2):124–8.
Tamminga WJ, Wemer J, Oosterhuis B, Weiling J, Wilffert B, de Leij LF, de Zeeuw RA, Jonkman JH. CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive-related gender differences. Eur J Clin Pharmacol. 1999;55(3):177–84.
Hagg S, Spigset O, Dahlqvist R. Influence of gender and oral contraceptives on CYP2D6 and CYP2C19 activity in healthy volunteers. Br J Clin Pharmacol. 2001;51(2):169–73.
U.S. Department of Health and Human Services, FDA. Guidance for Industry-Bioanalytical method validation. U.S. Department of Health and Human Services, FDA, CDER. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070107.pdf. Accessed 28 Apr 2015.
van Schaick EA, Lechat P, Remmerie BM, Ko G, Lasseter KC, Mannaert E. Pharmacokinetic comparison of fast-disintegrating and conventional tablet formulations of risperidone in healthy volunteers. Clin Ther. 2003;25(6):1687–99.
Snoeck E, Van Peer A, Sack M, Horton M, Mannens G, Woestenborghs R, Meibach R, Heykants J. Influence of age, renal and liver impairment on the pharmacokinetics of risperidone in man. Psychopharmacology. 1995;122:223–9.
Huang Mingzhu, Shen-Tu Jianzhong, Xingjiang Hu, Chen Junchun, Liu Jian, Lihua Wu. Comparative fasting bioavailability of dispersible and conventional tablets of Risperidone: a single-dose, randomized-sequence, open-label, two-period crossover study in healthy male Chinese volunteers. Clin Ther. 2012;34(6):1432–9.
Liu Yun, Zhang Meng-qi, Jia Jing-ying, Liu Yan-mei, Liu Gang-yi, Li Shui-jun, Wang Wei, Weng Li-ping, Chen Yu. Bioequivalence and pharmacokinetic evaluation of two formulations of risperidone 2 mg. Drugs R D. 2013;13(1):29–36.
Kelly DL, Conley RR, Tamminga CA. Differential olanzapine plasma concentrations by sex in a fixed-dose study. Schizophr Res. 1999;40(2):101–4.
Aichhorn W, Weiss U, Marksteiner J, Kemmler G, Walch T, Zernig G, Stelzig Schoeler R, Stuppaeck C, Geretsegger C. Influence of age and gender on risperidone plasma concentrations. J Psychopharmacol. 2005;19(4):395–401.
Cabaleiro T, Ochoa D, Lopez-Rodriguez R, Román M, Novalbos J, Ayuso C, Abad-Santos F. Effect of polymorphisms on the pharmacokinetics, pharmacodynamics, and safety of risperidone in healthy volunteers. Hum Psychopharmacol Clin Exp. 2014;29(5):459–69.
Almoguera B, Riveiro-Alvarez R, Lopez-Castroman J, Dorado P, Vaquero Lorenzo C, Fernandez-Piqueras J, Llerena A, Abad-Santos F, Baca-García E, Dal-Ré R, Ayuso C. Association of common genetic variants with risperidone adverse events in a Spanish schizophrenic population. Pharm J. 2013;13(2):197–204.
López-Rodríguez R, Cabaleiro T, Ochoa D, Román M, Borobia AM, Carcas AJ, Ayuso C, Novalbos J, Abad-Santos F. Pharmacodynamic genetic variants related to antipsychotic adverse reactions in healthy volunteers. Pharmacogenomics. 2013;14(10):1203–11.
Yong Chung J, Jung Lee Y, Bok Jang S, Ahyoung Lim L, Soo Park M, Hwan Kim K. CYP3A5*3 genotype associated with intrasubject pharmacokinetic variation toward tacrolimus in bioequivalence study. Ther Drug Monit. 2010;32(1):67–72.
Li Xiaobing, Shi Fuguo, He Xiaojing, Jian Lingyan, Ding Li. A rapid and sensitive LC-MS/MS method for determination of lercanidipine in human plasma and its application in a bioequivalence study in Chinese healthy volunteers. J Pharm Biomed Anal. 2016;128:67–72.
Acknowledgements
The authors thank all the volunteers who have participated in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This work was supported in part by the Nature Science Foundation of China (No. 81401113), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20130162120060), and Hunan Provincial Natural Science Foundation of China (No. 2017JJ3444).
Conflict of interest
The authors declare no conflict of interest in this study.
Ethical approval
The study was conducted in line with GCP, the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the School of Pharmaceutical Sciences, Central South University.
Informed consent
Informed consent was signed by all volunteers before the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Deng, Y., Yan, M. et al. Genotyping as a Key Element of Sample Size Optimization in Bioequivalence of Risperidone Tablets. Eur J Drug Metab Pharmacokinet 43, 431–439 (2018). https://doi.org/10.1007/s13318-017-0459-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-017-0459-1