Abstract
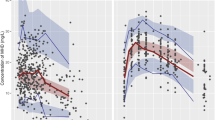
The aim of the study was to develop a population pharmacokinetic (PPK) model of oxcarbazepine and optimize the treatment of oxcarbazepine in Chinese patients with epilepsy. A total of 108 oxcarbazepine therapeutic drug monitoring samples from 78 patients with epilepsy were collected in this study. The pharmacologically active metabolite 10,11-dihydro-10-hydrocarbamazepine (MHD) was used as the analytical target for monitoring therapy of oxcarbazepine. Patients’ clinical data were retrospectively collected. The PPK model for MHD was developed using Phoenix NLME 1.2 with a non-linear mixed-effect model. MHD pharmacokinetics obeys a one-compartment model with first-order absorption and elimination. The effect of age, gender, red blood cell count, red blood cell specific volume, hemoglobin (HGB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and serum creatine were analyzed. Bootstrap and data splitting were used simultaneously to validate the final PPK models. The mean values of volume of distribution and clearance of MHD in the patients were 14.2 L and 2.38 L h−1, respectively. BUN and HGB influenced the MHD volume of distribution according to the following equation: V = tvV × (BUN/4.76)−0.007 × (HGB/140)−0.001 × eηV. The MHD clearance was dependent on ALT and gender as follows: CL = tvCL × (ALT/30)0.181 × (gender) × 1.083 × eηCL. The final PPK model was demonstrated to be suitable and effective and it can be used to evaluate the pharmacokinetic parameters of MHD in Chinese patients with epilepsy and to choose an optimal dosage regimen of oxcarbazepine on the basis of these parameters.



Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BUN:
-
Blood urea nitrogen
- Cr:
-
Serum creatine
- EIAEDs:
-
Enzyme-inducing antiepileptic drugs
- HCT:
-
Red blood cell specific volume
- HGB:
-
Hemoglobin
- IPRED:
-
Individual predicted value
- MHD:
-
10,11-Dihydro-10-hydrocarbamazepine
- PRED:
-
Predicted value
- RBC:
-
Red blood cell counts
References
Alves G, Figueiredo I, Castel-Branco M, Loureiro A, Fortuna A, Falcão A, Caramona M (2007) Enantioselective HPLC–UV method for determination of eslicarbazepine acetate (BIA 2-093) and its metabolites in human plasma. Biomed Chromatogr 21(11):1127–1134
Anderson GD, Hakimian S (2014) Pharmacokinetic of antiepileptic drugs in patients with hepatic or renal impairment. Clin Pharmacokinet 53(1):29–49
Bressolle F, Bromet-Petit M, Audran M (1996) Validation of liquid chromatographic and gas chromatographic methods. Applications to pharmacokinetics. J Chromatogr B Biomed Appl 686(1):3–10
Bring P, Ensom MH (2008) Does oxcarbazepine warrant therapeutic drug monitoring? A critical review. Clin Pharmacokinet 47(12):767–778
Flesch G (2004) Overview of the clinical pharmacokinetics of oxcarbazepine. Clin Drug Investig 24(4):185–203
Flesch G, Tudor D, Souppart C, D’Souza J, Hossain M (2002) Oxcarbazepine final market image tablet formulation bioequivalence study after single administration an at steady state in healthy subjects. Int J Clin Pharmacol Ther 40(11):524–532
Flesch G, Czendlik C, Renard D, Lloyd P (2011) Pharmacokinetics of the monohydroxy derivative of oxcarbazepine and its enantiomers after a single intravenous dose given as racemate compared with a single oral dose of oxcarbazepine. Drug Metab Dispos 39(6):1103–1110
Fortuna A, Alves G, Falcão A, Soares-da-Silva P (2010) Binding of licarbazepine enantiomers to mouse and human plasma proteins. Biopharm Drug Dispos 31(5–6):362–366
Hu GX, Qiu XJ, Zhou HY (2005) Determination of active metabolite of oxcarbazepine by RP-HPLC and study of its pharmacokinetics in Chinese healthy volunteers. Zhongguo Lin Chuang Yao Li Xue Za Zhi 3(21):209–212
Johannessen SI, Battino D, Berry DJ, Bialer M, Krämer G, Tomson T, Patsalos PN (2003) Therapeutic drug monitoring of the newer antiepileptic drugs. Ther Drug Monit 25(3):347–363
Juenke JM, Brown PI, Urry FM, McMillin GA (2006) Drug monitoring and toxicology: a procedure for the monitoring of oxcarbazepine metabolite by HPLC–UV. J Chromatogr Sci 44(1):45–48
Kim DW, Gu N, Jang IJ, Chu K, Yu KS, Cho JY, Yoon SH, Kim HS, Oh J, Lee SK (2012) Efficacy, tolerability, and pharmacokinetics of oxcarbazepine oral loading in patients with epilepsy. Epilepsia 53(1):e9–e12
Lameire N, Van Biesen W, Vanholder R (2005) Acute renal failure. Lancet 365(9457):417–430
Maia J, Almeida L, Falcão A, Soares E, Mota F, Potgieter MA, Potgieter JH, Soares-da-Silva P (2008) Effect of renal impairment on the pharmacokinetics of eslicarbazepine acetate. Int J Clin Pharmacol Ther 46(3):119–130
May TW, Korn-Merker E, Rambeck B (2003) Clinical pharmacokinetics of oxcarbazepine. Clin Pharmacokinet 42(12):1023–1042
Meibohm B, Beierle I, Derendorf H (2002) How important are gender differences in pharmacokinetics? Clin Pharmacokinet 41(5):329–342
Neels HM, Sierens AC, Naelaerts K, Scharpé SL, Hatfield GM, Lambert WE (2004) Therapeutic drug monitoring of old and newer anti-epileptic drugs. Clin Chem Lab Med 42(11):1228–1255
Northam RS, Hernandez AW, Litzinger MJ, Minecan DN, Glauser TA, Mangat S, Zheng C, Souppart C, Sturm Y (2005) Oxcarbazepine in infants and young children with partial seizures. Pediatr Neurol 33(5):337–344
Nunes T, Rocha JF, Falcão A, Almeida L, Soares-da-Silva P (2013) Steady-state plasma and cerebrospinal fluid pharmacokinetics and tolerability of eslicarbazepine acetate andoxcarbazepine in healthy volunteers. Epilepsia 54(1):108–116
Park KJ, Kim JR, Joo EY, Seo DW, Hong SB, Ko JW, Kim SR, Huh W, Lee SY (2012) Drug interaction and pharmacokinetic modeling of oxcarbazepine in Korean patients with epilepsy. Clin Neuropharmacol 35(1):40–44
Perucca E, Battino D, Tomson T (2014) Gender issues in antiepileptic drug treatment. Neurobiol Dis. doi:10.1016/j.nbd.2014.05.011
Rouan MC, Lecaillon JB, Godbillon J, Menard F, Darragon T, Meyer P, Kourilsky O, Hillion D, Aldigier JC, Jungers P (1994) The effect of renal impairment on the pharmacokinetics of oxcarbazepine and its metabolites. Eur J Clin Pharmacol 47(2):161–167
Wegner I, Wilhelm AJ, Sander JW, Lindhout D (2013) The impact of age on lamotrigine and oxcarbazepine kinetics: a historical cohort study. Epilepsy Behav 29(1):217–221
Acknowledgments
This work was supported by the Pharmaceutical foundation of Suzhou (No. SYSD2012129 and SYSD2013144).
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s13318-016-0379-5.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, Y., Zhang, Q., Xu, W. et al. Population pharmacokinetic modeling of oxcarbazepine active metabolite in Chinese patients with epilepsy. Eur J Drug Metab Pharmacokinet 41, 345–351 (2016). https://doi.org/10.1007/s13318-015-0266-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-015-0266-5