Abstract
Introduction
This study aims to define the distribution of direct healthcare costs for people with diabetes treated in two healthcare regions in Italy, based on number of comorbidities and treatment regimen.
Methods
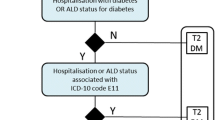
This was a retrospective analysis using data from two local health authority administrative databases (Campania and Umbria) in Italy for the years 2014–2018. Data on hospital care, pharmaceutical and specialist outpatient and laboratory assistance were collected. All people with diabetes in 2014–2018 were identified on the basis of at least one prescription of hypoglycemic drugs (ATC A10), hospitalization with primary or secondary diagnosis of diabetes mellitus (ICD9CM 250.xx) or diabetes exemption code (code 013). Subjects were stratified into three groups according to their pharmaceutical prescriptions during the year: Type 1/type 2 diabetes (T1D/T2D) treated with multiple daily injections with insulin (MDI), type 2 diabetes on basal insulin only (T2D-Basal) and type 2 diabetes not on insulin therapy (T2D-Oral).
Results
We identified 304,779 people with diabetes during the period for which data was obtained. Analysis was undertaken on 288,097 subjects treated with glucose-lowering drugs (13% T1D/T2D-MDI, 13% T2D-Basal, 74% T2D-Oral). Average annual cost per patient for the year 2018 across the total cohort was similar for people with T1D/T2D-MDI and people with T2D-Basal (respectively €2580 and €2254) and significantly lower for T2D-Oral (€1145). Cost of hospitalization was the main driver (47% for T1D/T2D-MDI, 45% for T2D-Basal, 45% for T2D-Oral) followed by drugs/devices (35%, 39%, 43%) and outpatient services (18%, 16%, 12%). Average costs increased considerably with increasing comorbidities: from €459 with diabetes only to €7464 for a patient with four comorbidities. Similar trends were found across all subgroups analysis.
Conclusion
Annual cost of treatment for people with diabetes is similar for those treated with MDI or with basal insulin only, with hospitalization being the main cost driver. This indicates that both patient groups should benefit from having access to scanning continuous glucose monitoring (CGM) technology which is known to be associated with significantly reduced hospitalization for acute diabetes events, compared to self-monitored blood glucose (SMBG) testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Treatment costs for people with diabetes in two healthcare regions in Italy are similar for those treated with MDI (T1D/T2D-MDI) or with basal insulin only (T2D-Basal). |
For people with T1D or T2D treated with insulin the main cost driver is hospitalization, followed by costs for drugs and devices. |
When all direct costs are considered, during the period 2014–2018, our analysis calculates the overall annual direct costs per person with diabetes as €1229 in Umbria and €1495 in Campania. |
Per-patient costs of diabetes treatment increase incrementally with each additional comorbidity, with costs for a person with diabetes and four or more comorbidities being three to fourfold higher than for a person with no comorbidities. |
The use of scanning continuous glucose monitoring (CGM) could positively impact the most significant treatment costs for people with diabetes in Italy. |
Introduction
Diabetes is a complex metabolic disorder with a higher risk of microvascular or macrovascular complications compared to the general population, which is associated with increased mortality [1, 2]. In high-income countries, diabetes is one of the leading causes of cardiovascular disease, blindness, kidney failure, and lower limb amputation, with a substantial clinical and economic impact [3]. The need to implement glucose-lowering treatment to reduce long-term complications must be counterbalanced with the shorter-term risk of hypoglycemia [4].
In Italy, the ARNO Diabetes Observatory identifies people with diabetes through diabetes registries, documented prescriptions and hospital admissions. Data from the 2018 calendar year indicates an overall prevalence of diabetes in Italy at 6.2% (6.5% in men, 5.9% in women) [5]. Compared with people without diabetes, people with diabetes in Italy received 30% more prescription drugs, 20% more outpatient specialist services and were 86% more likely to be hospitalized [5]. National and international guidelines on medical care of diabetes in Italy are not well implemented, with consequent increases in the rate of diabetes-related disease and economic costs [5, 6]. This is compounded by low adherence to diabetes therapy in Italy. The second civic survey on diabetes in Italy from 2021 [7] indicates that adherence to diabetes therapy as prescribed, 1 year after starting, is 37.6% among men and 31.6% for women. Regional data show that treatment adherence rates in Campania are 35.9% for men and 30.9% for women. In Umbria, adherence rates are 34.7% and 32.8% in men and women, respectively. Similarly, for people with diabetes on insulin regimens in Italy, only 63% reported performing at least one daily self-monitoring of blood glucose (SMBG) test, with similar regional figures in Campania and Umbria [8]. With this background, the burden of care for people with diabetes in Italy is considerable. For 2018, the direct cost of medical services was estimated as €2833 per person with diabetes, the main cost drivers being hospitalization (€1152, 40.7%) and prescribed drugs (€1116, 39.4%) [5]. Overall, the annual direct cost of diabetes treatment to the Italian healthcare services has been estimated as between €8.1 and €9.6 billion [9, 10], of which €1.3 billion can be attributed to the cost of SMBG [10].
International guidelines currently recommend SMBG as the reference tool for glucose monitoring in people with diabetes treated with insulin, with recommendations that up to 10 finger-prick tests daily are required for effective glucose management [11]. However, there are barriers related to the use of SMBG [12] that have highlighted the need for new innovations in glucose monitoring, which can improve outcomes for people with diabetes and also modify the cost of care. This could include the use of scanning continuous glucose monitoring (CGM), which is proven to reduce HbA1c compared to SMBG testing for people with T1D or with T2D on insulin therapy [13,14,15], as well as reducing time in hypoglycemia [16, 17]. Use of scanning CGM is also associated with a significantly reduced rate of hospitalization for acute diabetes events (ADEs), such as diabetes ketoacidosis (DKA), for adults with T1D or T2D on insulin therapy in the 2-year period after starting, compared to using SMBG testing [18,19,20]. Such outcomes would predict long-term health economic benefits at a national level.
In Italy, eligibility and budget for scanning CGM sensors are defined at a regional level, which results in considerable variation in budget allocation for scanning CGM by regions across Italy. To date, scanning CGM systems were approved for reimbursement in the Umbria region in December 2016 for people with T1D or T2D, more precisely for:
-
All people with T1D
-
T1D and T2D with pregnancy or planned pregnancy and gestational diabetes mellitus (GDM)
-
T2D with at least one of the following conditions:
-
HbA1c 10 mmol/mol above target for more than 6 months
-
Repeated hypoglycemia
-
Reimbursement for scanning CGM was approved in the Campania region in February 2017 for people with T1D or T2D as indicated:
-
All people with T1D
-
People with T2D using ≥ 4 SMBG tests per day
The study reported here aims to estimate the number of people with diabetes in two healthcare regions in Italy, who are using intensive MDI regimens, compared with basal-only insulin or non-insulin regimens, and to quantify the direct healthcare costs associated with the management of diabetes in these treatment groups. We have identified areas of cost that may be reduced with the application of scanning or streaming CGM technology in each treatment population. In order to emphasize the comparative costs for specific diabetes drug treatment regimens, we have not included data on the treatment costs for people with diabetes but untreated with antidiabetic drugs. Such real-world data can be leveraged to show the direct burden of treating diabetes in a large cohort of patients in the Italian real-world setting, in terms of clinical and economic consequences. Together, the regions of Campania and Umbria are large enough to give a realistic reflection of the diabetes population in Italy, as they represent approximately 10% of the Italian population. Campania and Umbria report a diabetes prevalence of 5.9% and 5.2% respectively, which is lower than the national prevalence of 6.2% for Italy [5].
Methods
Data Source
The analysis was carried out using data extracted from the healthcare administrative databases of the Local Health Unit Umbria 2 and the Campania region, covering the period 2014–2018. For the purpose of providing the most current calendar-year cost-impact analysis we focused our analysis on the real-world healthcare resource utilization for 2018. Datasets were made available following the scientific agreement between CEIS (University of Tor Vergata) and Campania region/LHU Umbria 2. Exchange of data was compliant with the General Data Protection Regulation (EU) 2016/679 (GDPR).
For both regions, data was extracted from (1) Hospital Information System (SDO), which collects all hospitalization events across all public and private healthcare institutes in the region. Discharge records include the patient demographic and clinical information, including principal diagnostic and secondary discharge codes as set out in the International Classification of Diseases (ICD-9-CM); (2) Outpatient Visits and Diagnostic (SIAS), in which all the specialist services performed on an outpatient basis, the maternal-infant consultancy activities and the instrumental and laboratory diagnostics services are recorded, as well as all the services, both diagnostic and therapeutic, provided by the nomenclator referred to in Ministerial Decree 22.7.96 and subsequent amendments and additions, carried out at accredited public and private outpatient facilities; (3) Pharmaceutical services databases, which contain information about all drugs reimbursed by the healthcare system and dispensed by hospital, municipal and private pharmacies.
Cohort Definitions
People with diabetes were identified by selecting all patients with at least one prescription of hypoglycemic drugs (ATC A10) or hospitalization with diabetes mellitus as a primary or secondary diagnosis (ICD9CM 250) or a medical exemption certificate code (code 013) for any type of diabetes. The selected subjects were stratified according to their pharmaceutical prescriptions during the year as described in Table 1. No age-related inclusion criteria were applied.
Cost-Impact Modelling
As this study was conducted on healthcare administrative databases covering the entire populations of Umbria and Campania for the calendar year 2018, no statistical tests were required based on sampling as costs for all individuals with T1D or T2D were available. Average per-patient costs for each diabetes type and treatment modality, including hospital admission criteria, could be calculated and compared across the study period.
For the selected patients, the cost for treatment was estimated by selecting all hospital admissions, consumption of drugs and specialist outpatient services during the year. Ordinary and day hospital admissions were costed according to the regional tariff for acute care services and pharmacological treatments acquisition costs for regional healthcare services were considered. Outpatient specialist services and laboratory tests were costed according to tariffs made available for regional outpatient specialist assistance services.
In addition, the occurrence and economic burden of admission to hospital for ADEs was considered for hypoglycemia, DKA coma and hyperglycemia, as defined in Table 2. The presence of comorbidities was verified through disease exemptions and by analyzing hospitalizations, drug use and specialist services that occurred during the 2014–2018 period. The comorbidity selection criteria are summarized in Table 3.
Ethical Approval
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Internal review board approval was not sought as this analysis does not contain any studies with human participants or animals performed by any of the authors.
Results
Patient Cohorts and Overall Costs
Across the two health authorities we identified 304,779 people with a diagnosis of diabetes, of whom 288,097 patients were being treated with antidiabetic drugs in 2018 (Table 4). Of these 24,710 were from Umbria and 263,387 were from Campania. Amongst this total cohort, 37,333 (13.0%) were people with T1D/T2D-MDI, 36,450 (12.7%) were people with T2D-Basal and 214,314 (74.4%) were people with T2D-Oral. Average annual cost per patient across the total cohort was similar for the T1D/T2D-MDI and T2D-Basal cohorts (respectively €2580 and €2254) and significantly lower for the T2D-Oral cohort (€1145). This pattern was also reflected in the individual costs of treatment in the separate regions, with a slightly lower per-patient cost for treatment in Umbria compared to Campania (Table 4).
Overall Costs of Care for People with Diabetes
The costs of drug prescriptions and delivery, specialist outpatient care and hospitalizations for the cohort of people with diabetes in Umbria and Campania health authorities are summarized in Table 5 and Supplementary Table S1, along with the aggregate costs for the total patient cohort. The average treatment cost per patient in Umbria and Campania (Fig. 1) was €1229 and €1495, respectively, with higher costs for the T1D/T2D-MDI treatment groups (€2266 and €2621 respectively) or the T2D-Basal treatment groups (€1979 and €2272 respectively) compared to T2D-Oral treatment group (€891 and €1169 respectively).
The average cost per patient increases according to the number of comorbidities (Fig. 1, Table 5, Supplementary Table S1). For the T1D/T2D-MDI treatment groups, patients in Umbria and Campania without comorbidities have treatment costs of €735 and €781, respectively, rising to €5639 and €17,922 for patients with four or more comorbidities (Fig. 1a, Fig. 1b). The T2D-Basal treatment groups had similarly low treatment costs when no comorbidities were present, rising progressively to €4154 (Umbria) and €6078 (Campania) for four or more comorbidities. The T2D-Oral group showed a similar pattern in each region, although costs did not escalate to the same level as for the T1D/T2D-MDI and T2D-Basal treatment groups. For patients with one or no comorbidities, the most significant costs are for drug prescriptions and delivery, whereas as the number of comorbidities increases the costs of hospitalizations become the most significant cost of care (Fig. 1, Table 5, Supplementary Table S1).
Distribution of Costs of Care for People with Diabetes on Different Treatment Regimens
Average treatment costs for people with diabetes increased considerably as the number of comorbidities increased (Table 6, Supplementary Table S2). Across the total cohort of patients the per-person treatment cost for those without comorbidities was €459, rising to €7464 for a person with diabetes and four or more comorbidities.
For people in the T1D/T2D-MDI treatment group the pattern of costs was similar across Umbria and Campania regions for people with fewer than four comorbidities (Fig. 1a, b). Drug costs were most significant for people with one or no comorbidities, whereas costs for hospitalization were largest for those with two or three comorbidities. For Umbria, patients with diabetes and four or more comorbidities had hospitalization as the major cost of care, whereas the Campania region registered drug costs as being the most significant cost (63.8% of total cost) for this treatment group. However, the small number of patients with four or more comorbidities in Campania (n = 165, 0.5%) was also accompanied by a much larger per-patient cost in this cohort (€17,922), and the small number of patients in the T1D/T2D-MDI group in Umbria (n = 37, 0.9%) means that a meaningful comparison between people with four or more comorbidities is impractical.
For people in the T2D-Basal treatment group the pattern of costs was similar across Umbria and Campania regions, with progressive increases at each level of comorbidity (Fig. 1). Drug costs were most significant for people with one or no comorbidities, drug and hospital costs were equally significant for people with two comorbidities, and costs for hospitalization were largest for people with three or more comorbidities.
Treatment costs for the T1D/T2D-MDI group and the T2D-Basal group were comparable in both Umbria and Campania, with increasing costs per patient with rising numbers of comorbidity, with the exception of the T1D/T2D-MDI group in Campania, as indicated above.
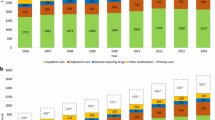
Per-patient treatment costs for the T2D-Oral patient group were lower than in the two insulin-treated groups (Table 4). In this context, the overall distribution of patients amongst the treatment groups is shown in Fig. 2a, compared to the distribution of treatment costs (Fig. 2b). Cost distribution was comparable with the insulin-treated cohorts, with the emphasis on drug costs at low levels of comorbidity, with the cost of hospitalization becoming the major factor as comorbidities increased.
Hospital admission for ADEs amongst the treatment groups, related to hypoglycemia, DKA, coma and hyperglycemia, was assessed according to ICD9 codes. In both regions, hospitalization for at least one ADE was significant in the T1D/T2D-MDI and T2D-Basal cohorts, with increasing prevalence at greater numbers of comorbidities (Fig. 3a–d). ADEs resulting in hospital admission were much less frequent in the T2D-Oral treatment cohort (Fig. 3e, f).
Modelling of Diabetes Treatment Costs in Italy
On the basis of the treatment costs available for Umbria and Campania in 2018, we modelled the total treatment costs for the population of people with diabetes in Italy (Table 7, Supplementary Table S2). The total number of people with a diagnosis of diabetes in Italy was taken to be 3,483,860, which was previously identified through linkage of the Marche healthcare authority [9]. On the basis of the data for Umbria and Campania, 79.21% of people with a diagnosis of diabetes were on antidiabetic treatment regimens in Umbria, and the corresponding proportion for Campania was 96.27%. Thus, treatment costs for Italy were modelled for 2,759,566 patients (based on Umbria) and for 3,353,912 patients (based on Campania).
In both scenarios, treatment costs are driven primarily by the cost of hospitalization, followed by drug costs (Table 7, Supplementary Table S2, Fig. 4). Thus, based on the model extrapolated from the Umbria data, the total direct costs for diabetes in Italy would be €3.39 billion, whereas the model populated using the Campania data would be €5.01 billion. The model based on Umbria puts the annual cost of hospitalization for all treatment groups at €1.43 billion, with €1.34 billion spent on prescription drugs. Based on the Campania data, the annual cost of hospitalization is €2.28 billion, with drug treatment costs of €2.04 billion. As with the separate data from Umbria and Campania, the proportion of costs for hospitalization in the model for Italy increases as the number of comorbidities rises.
Discussion
Our analysis of the annual cost of treatment for people with diabetes across two healthcare regions in Italy, show that treatment costs are similar for those treated with MDI (T1D/T2D-MDI) or with basal insulin only (T2D-Basal). In both treatment cohorts, the main cost driver is hospitalization. When all direct costs are considered, the overall cost of diabetes treatment in Italy can be estimated as €3.4–5.0 billion. Our analysis calculates the overall annual direct costs per person with diabetes in Umbria as €1229, and €1495 for Campania. Both of these figures are below previous estimated per-person treatment costs in Italy, which have a calculated €2318 [9] or €2833 [5] per-person cost. As a consequence, the direct treatment costs for Italy, modelled on this data, are lower than previous estimates. However, we have included here only direct treatment costs associated with diabetes. When that constraint is applied to one of the earlier studies [9], the total annual treatment cost in Italy is estimated as €3.7 billion, which is comparable with our study.
Diabetes treatment is complicated by the presence of one or more comorbid conditions, including cardiac disease, retinopathy, nephropathy and neuropathic disease. Our data show that the per-patient costs of diabetes treatment increase incrementally with each additional comorbidity, with costs for a person with diabetes and four or more comorbidities being three or fourfold higher than for a person with no comorbidities. In our analysis, although hospital admission was the most significant cost driver, it was a lower proportion of costs compared to a previous analysis of diabetes treatment costs and comorbidities [9], based on the Marche region which indicated that hospitalization accounted for 59% of treatment costs for a person with diabetes alone, rising to 80% of costs for a person with diabetes and three or four comorbidities. Our analysis shows hospitalization accounts for only 6% (Umbria) or 11% (Campania) of costs for a person with diabetes alone, rising to 53% of costs in both regions for a person with diabetes and three or more comorbidities. This includes the costs for hospital admission for ADEs such as DKA, hypoglycemia, coma and hyperglycemia.
The emphasis on hospitalization as the predominant cost driver is of interest, since it is known that this is a modifiable cost with the use of glucose-sensing technologies. Continuous glucose monitoring (CGM) is an accepted alternative to daily finger prick testing and is proven to improve outcomes for people with diabetes, when compared to SMBG testing [13, 21,22,23]. To date, among CGM systems the scanning CGM sensor has had the most advantageous acquisition cost and can be used as a replacement for daily SMBG tests for adults and children (aged 4 and older) with type 1 diabetes (T1D) or type 2 diabetes (T2D) and for use in GDM. The system is factory calibrated [24], with no need for daily reference SMBG calibration by the person with diabetes, and it can also be used to make insulin-dosing decisions without the need for an adjunct SMBG test.
It has been shown previously that the use of scanning CGM is associated with substantial reductions in hospital admissions for ADEs, both for people with T1D or with T2D. The RELIEF study, using data from the national French claims database (SNDS), reported that in the 12 months after starting to use scanning CGM, people with T1D had a 49.0% reduction in hospital admissions for ADEs [18], and people with T2D had 39.4% fewer admissions for ADEs [18]. The reductions, both in T1D and in T2D, were driven by a lower incidence of hospitalization for DKA and were maintained at 24 months after starting scanning CGM [19]. The RELIEF study has also confirmed that the use of scanning CGM in people with T2D on basal insulin treatment is also associated with significantly reduced rates of hospital admission for DKA and severe hypoglycemia, for at least 2 years after initiation of scanning CGM [20]. Other studies have shown that using scanning CGM compared to SMBG is associated with a 25–40% reduction in all-cause hospital admissions for people with T1D [25, 26] and a 11–15% reduction in all-cause admissions for people with T2D treated with insulin [25, 27]. Given that the costs of hospitalization can be as high as €4835 for each person with diabetes and significant comorbid disease in Italy (Table 5, Supplementary Table S1), such reductions in hospital admission can be substantial. Further studies are needed to better understand the relationship between use of scanning CGM or other CGM systems in healthcare utilization for cardiovascular disease, neuropathy, nephropathy and retinopathy in this context, all of which are comorbid complications of diabetes.
An additional cost-saving benefit of using scanning CGM is that the daily use of SMBG test strips for people with T1D or T2D on MDI and for people with T2DM on basal-only insulin therapy is considerably reduced [18,19,20]. For people with T1D or T2D, an 80% fall in daily test strip use has been documented in the 2 years after starting scanning CGM [19], with a 35% reduction in daily test strip use for people with T2D on basal insulin only therapy [19]. The estimated annual cost of using SMBG for daily glucose testing in Italy is €1.3 billion as a direct treatment cost of diabetes [9], which constitutes a considerable proportion of the total overall cost of diabetes care for Italy. The potential cost savings of using scanning CGM are therefore persuasive.
Overall, poor treatment adherence in Italy leads to lower health-related outcomes and increased costs [7, 8]. At least one study investigating newer models of care in diabetes in Italy [28] has concluded that people with diabetes need straightforward treatment plans that can address their diabetes needs at any time and that fit with their real-world daily lives. Use of scanning CGM can provide a solution for daily glucose monitoring that meets these needs.
Limitations and Strengths
As with all modelling strategies, our study has limitations and strengths. A first limitation is that we have assumed that the data from the Umbria and Campania regions are representative of the national landscape of diabetes and diabetes care. However, a strength of our analysis is that together the Umbria and Campania regions cover approximately 10% of the Italian population, which gives us confidence that we are dealing with a representative population. However, we accept that regional diversity across Italy may include variations in healthcare utilization that make this a speculative assumption. A second limitation is that we cannot confirm that diabetes management at regional and national levels has been consistent or compliant with national guidelines, as might be inferred by the differential in the number of untreated patients between Umbria and Campania. Lastly, an additional limitation pertains to the study period of 2014–2018, which may result in an underestimation of costs. While the national reimbursement rates for the healthcare services in question have remained constant up until 2023, this limitation can be attributed to the exclusion of new technologies approved after 2018. Strengths of our study are the large size of the patient cohort analyzed and the detailed treatment and prescribing codes that have allowed us to define separate diabetes treatment groups within the total population and assign real direct costs of care.
Conclusions
Our study is the first to make a detailed analysis of the annual direct treatment costs for people in Italy with diabetes on different antidiabetic therapeutic regimens, including intensive insulin therapy with MDI, non-intensive basal insulin therapy and non-insulin therapy. We show that treatment costs are similar for those treated with MDI (T1D/T2D-MDI) or with basal insulin only (T2D-Basal) and substantially higher than for people with T2D not on insulin therapy (T2D-Oral). In the T1D/T2D-MDI and T2D-Basal treatment cohorts, the main cost driver is hospitalization and the per-patient cost of treatment increases significantly as the number of comorbid conditions rises. The costs of diabetes treatment indicate that people with T2D on MDI or basal insulin only regimens in Italy can benefit from having access to a glucose-sensing technology which is known to be associated with reduced hospitalization for acute diabetes events.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Nathan DM, Bayless M, Cleary P, et al. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62:3976–86.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. New Engl J Med. 2008;359:1577–89.
American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917–28.
Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2021;64:2609–52.
Bonora E, Cataudella S, Marchesini G, et al. Clinical burden of diabetes in Italy in 2018: a look at a systemic disease from the ARNO Diabetes Observatory. BMJ Open Diabetes Res Care. 2020;8:e001191. https://doi.org/10.1136/bmjdrc-2020-001191.
Bonora E, Cataudella S, Marchesini G, et al. A view on the quality of diabetes care in Italy and the role of Diabetes Clinics from the 2018 ARNO Diabetes Observatory. Nutr Metab Cardiovasc Dis. 2020;30:1945–53.
Inglese SA. Disuguaglianze, territorio, prevenzione, un percorso ancora lungo: Seconda Indagine civica sul Diabete. 2021. https://www.cittadinanzattiva.it/multimedia/import/files/comunicati/salute/Seconda_Indagine_civica_sul_Diabete_Abstract_def.pdf. Accessed 27 Mar 2024.
Aprile V, Baldissera S, D’Argenzio A, et al. I risultati nazionali dello studio QUADRI (QUalità dell’Assistenza alle persone Diabetiche nelle Regioni Italiane). Roma: Istituto Superiore di Sanità; 2007. (Rapporti ISTISAN 07/10).
Marcellusi A, Viti R, Sciattella P, et al. Economic aspects in the management of diabetes in Italy. BMJ Open Diabetes Res Care. 2016;4(1):e000197. https://doi.org/10.1136/bmjdrc-2016-000197.
Marcellusi A, Viti R, Mecozzi A, Mennini FS. The direct and indirect cost of diabetes in Italy: a prevalence probabilistic approach. Eur J Health Econ. 2016;17:139–47.
American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, et al. 7. Diabetes Technology: Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45:S97–112.
Ward JEF, Stetson BA, Mokshagundam SPL. Patient perspectives on self-monitoring of blood glucose: perceived recommendations, behaviors and barriers in a clinic sample of adults with type 2 diabetes. J Diabetes Metab Disord. 2015;14:43. https://doi.org/10.1186/s40200-015-0172-z.
Leelarathna L, Evans ML, Neupane S, et al. Intermittently scanned continuous glucose monitoring for type 1 diabetes. N Engl J Med. 2022;387(16):1477–87.
Jendle J, Eeg-Olofsson K, Svensson A-M, Franzen S, Lamotte M, Levrat-Guillen F. Cost-effectiveness of the FreeStyle Libre® system versus blood glucose self-monitoring in individuals with type 2 diabetes on insulin treatment in Sweden. Diabetes Ther. 2021;12:3137–52.
Nathanson D, Svensson A-M, Miftaraj M, Franzén S, Bolinder J, Eeg-Olofsson K. Effect of flash glucose monitoring in adults with type 1 diabetes: a nationwide, longitudinal observational study of 14,372 flash users compared with 7691 glucose sensor naive controls. Diabetologia. 2021;64:1595–1603.
Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388:2254–63.
Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline J-P, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8:55–73.
Roussel R, Riveline J-P, Vicaut E, et al. Important drop in the rate of acute diabetes complications in people with type 1 or type 2 diabetes after initiation of flash glucose monitoring in France: the RELIEF study. Diabetes Care. 2021;44(6):1368–76.
Riveline J-P, Roussel R, Vicaut E, et al. Reduced rate of acute diabetes events with flash glucose monitoring is sustained for two-years after initiation: extended outcomes from the RELIEF study. Diabetes Technol Ther. 2022;24(9):611–8.
Guerci B, Roussel R, Levrat-Guillen F, et al. Important decrease in hospitalizations for acute diabetes events following FreeStyle Libre® system initiation in people with type 2 diabetes on basal insulin therapy in France. Diabetes Technol Ther. 2023;25(1):20–30.
Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317:371–8.
Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections. Ann Intern Med. 2017;167:365–74.
Martens T, Beck RW, Bailey R, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin. JAMA. 2021;325:2262–72.
Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol. 2015;9:209–14.
Reaven PD, Newell M, Rivas S, Zhou X, Norman GJ, Zhou JJ. Initiation of continuous glucose monitoring is linked to improved glycemic control and fewer clinical events in type 1 and type 2 diabetes in the veterans health administration. Diabetes Care. 2023;46:854–63.
Mustonen J, Rautiainen P, Lamidi M-L, Lavikainen P, Martikainen J, Laatikainen T. The use of isCGM leads to marked reduction in severe hypoglycemia requiring emergency medical service or hospital admission and diabetic ketoacidosis in adult type 1 diabetes patients. Acta Diabetol. 2023;60:891–98.
Miller E, Kerr MSD, Roberts GJ, Nabutovsky Y, Wright E. Flash CGM associated with event reduction in nonintensive diabetes therapy. Am J Manag Care. 2021;27:e372–7.
Regina RL, Pandolfi D, Stabile N, et al. A new case manager for diabetic patients: a pilot observational study of the role of community pharmacists and pharmacy services in the case management of diabetic patients. Pharmacy (Basel). 2020;8:193.
Medical Writing, Editorial and Other Assistance.
Medical writing and editorial assistance in the preparation of this article was provided by Dr Robert Brines of Bite Medical Consulting. Support for this assistance was funded by Abbott Diabetes Care.
Authorship.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
The authors acknowledge funding support from Abbott Diabetes Care who supported the Rapid Service Fee. The funding did not affect the collection, analysis, or presentation of the data.
Author information
Authors and Affiliations
Contributions
Francesco Saverio Mennini is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All the authors contributed to the methodology, the analyses and the manuscript writing.
Corresponding author
Ethics declarations
Conflict of Interest
Fleur Levrat-Guillen is employed by Abbott Diabetes Care. All other authors have no conflict of interest.
Ethical Approval
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Internal review board approval was not sought as this analysis does not contain any studies with human participants or animals performed by any of the authors. Datasets were made available following the scientific agreement between CEIS (University of Tor Vergata) and Campania region/ LHU Umbria 2. Exchange of data was compliant with the General Data Protection Regulation (EU) 2016/679 (GDPR).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mennini, F.S., Sciattella, P., Marcellusi, A. et al. An Analysis of the Distribution of Direct Cost of Diabetes Care in Selected Districts in Italy. Diabetes Ther 15, 1417–1434 (2024). https://doi.org/10.1007/s13300-024-01580-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-024-01580-z