Abstract
Introduction
The effect of dipeptidyl peptidase-4 (DDP-4) inhibitors versus α-glucosidase inhibitors (AGIs) on the treatment of type 2 diabetes mellitus (T2DM) in a real-world setting is unknown. The aim of this real-world study was to compare the glucose-lowering effect and tolerability of vildagliptin as add-on to metformin monotherapy (VM) and AGI as add-on to metformin monotherapy (AM) in Chinese patients with T2DM.
Methods
This was a subgroup analysis of the China Prospective Diabetes Study, a post-marketing, prospective, observational, real-world study conducted at 52 centers in China. T2DM patients with inadequate glycemic control on metformin monotherapy who received VM or AM were included. The composite primary endpoint was glycemic control (hemoglobin A1c [HbA1c] < 7%) after 12 months in the absence of tolerability events (hypoglycemia, weight gain ≥ 3%, or gastrointestinal events leading to treatment discontinuation). Propensity score matching (PSM) was used to balance the two groups.
Results
The success rates of the composite endpoint were higher in the VM group (n = 604/159 before/after PSM) than in the AM group (n = 159/157 before/after PSM), but the difference was not statistically significant (before PSM: 53.0 vs. 46.5%, P = 0.148; after PSM: 56.7 vs. 45.9%, P = 0.055). The glycemic control rate and HbA1c reduction were similar between groups at 3, 6, and 12 months. Compared with the AM group, the VM group had lower risks of any tolerability event (relative risk [RR] 0.53, 95% confidence interval [CI] 0.33–0.83, P = 0.006), of any adverse event (AE) (RR 0.64, 95% CI 0.41–1.00, P = 0.049), and of any serious AE (RR 0.45, 95% CI 0.25–0.81, P = 0.007).
Conclusion
The results of this real-world study suggest that vildagliptin as add-on to metformin monotherapy had a similar glucose-lowering effect to AGI as add-on to metformin monotherapy, but with better safety.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Randomized trials have shown that dipeptidyl peptidase-4 (DPP-4) inhibitors and α-glucosidase inhibitors (AGIs) are effective therapies for type 2 diabetes (T2DM). Nevertheless, real-world data are missing regarding the effects of DPP-4 inhibitors versus AGIs as add-ons to metformin monotherapy in Chinese T2DM patients. |
The aim of this study was to compare the glucose-lowering effect and tolerability of vildagliptin as add-on to metformin monotherapy (VM) and AGI as add-on to metformin monotherapy (AM) in Chinese T2DM patients in a real-world setting. |
What was learned from the study? |
T2DM patients prescribed VM were younger, more obese and had a higher baseline glycated hemoglobin (HbA1c) and a shorter duration of T2DM compared with T2DM patients prescribed AM in China. |
VM had a similar glucose-lowering effect as AM, but with better tolerability and safety. |
This study provides real-world evidence for physicians for treatment regimen selection, especially for Asian T2DM patients who differ from their Western counterparts in terms of genetic and dietary backgrounds. |
Introduction
The incidence and prevalence of type 2 diabetes mellitus (T2DM) have increased sharply in China over the past decades, such that the management of this disease has become an important healthcare challenge [1]. The overall glycemic control rate in Chinese T2DM patients has been reported to be around 47%, which is unsatisfactory [2]. At present, metformin is the standard first-line oral antidiabetic drug (OAD) for T2DM patients, but treatment intensification is usually required due to the progressive nature of T2DM. International and Chinese guidelines [3,4,5] recommend lifestyle intervention and combination therapy in newly diagnosed T2DM patients whose glycemic control is inadequate on monotherapy.
The selection of combination therapy should be individualized based on efficacy, tolerance, safety, and cost [3,4,5,6]. East Asians differ from Westerners in terms of diet, environment, and genetics and, consequently, special considerations regarding T2DM treatment have to be taken into account [7]. Carbohydrates are the core food for most Chinese individuals and, combined with genetic differences, the postprandial blood glucose levels are higher in the Chinese population than in the European population [8]. Since α-glucosidase inhibitors (AGIs) can reduce postprandial glucose levels by inhibiting the absorption of carbohydrates in the upper part of the small intestine, they are widely used to treat T2DM in Chinese patients [7]. Nevertheless, randomized controlled trials (RCTs) have shown that AGI use is associated with increased risks of gastrointestinal events, such as abdominal distension and flatulence [5]. Dipeptidyl peptidase-4 (DPP-4) inhibitors inhibit the catabolism of glucagon-like peptide-1 (GLP-1) by prolonging its plasma half-life and duration of action [9]. Meta-analyses have demonstrated that the different DPP-4 inhibitors have significant glucose-lowering effects [10], especially in Asians [11], probably due to the lower body mass index (BMI) of Asians relative to Westerners.
A number of RCTs have revealed the efficacy and safety of AGIs or DPP-4 inhibitors as monotherapy as well as in combination with metformin [12,13,14,15,16]. Similar glycemic reductions were observed when using DPP-4 inhibitors or AGIs although the mechanisms of these two types of drug are different. In addition, DPP-4 inhibitors have shown better tolerability and safety in RCTs. Nevertheless, real-world data are missing on the effects of DPP-4 inhibitors versus AGIs as add-ons to metformin monotherapy in Chinese T2DM patients. Vildagliptin is a potent DPP-4 inhibitor that has been shown to be well tolerated and efficacious in patients with T2DM [17].
The aim of this study was to compare the glucose-lowering effect and tolerability of vildagliptin as add-on to metformin (VM) and AGI as add-on to metformin (AM) in T2DM patients with inadequate glycemic control on metformin monotherapy by using data from the China Prospective Diabetes Study (PDS).
Methods
Study Design and Population
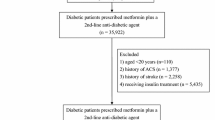
The study reported here was a subgroup analysis of the original China PDS, which was a post-marketing, multicenter, prospective, observational real-world study of patients with T2DM recruited from 52 sites in China between June 2013 and April 2017 [18]. The present study was conducted in compliance with the Declaration of Helsinki of 1964 and its later amendments. The study protocol was independently approved by the ethics committee/institutional review board of Chinese PLA General Hospital (central committee) and each participating study center (Electronic Supplementary Material [ESM] Table 1). Written informed consent for the study was obtained from all participants before enrollment. Since no individual person’s data were included in this article, the requirement for patient consent for publication was waived by the ethics committees.
The inclusion criteria for the PDS were: (1) ≥ 18 years of age; (2) diagnosis of T2DM with the last available (within 3 months) hemoglobin A1c (HbA1c) measurement ranging from 7 to 11%; (3) inadequate disease control on monotherapy; and (4) prescription of add-on OAD therapy by treating physician. The exclusion criteria were: (1) participation in any RCT; (2) treatment with insulin and/or GLP-1 analogue or agonist, and/or oral anti-diabetic single pill combination, or plans to use any of the above drugs before enrollment; (3) pregnant or lactating; or (4) unable to attend regular follow-up visits, including non-local residents or other patients, as judged by the investigators.
In order to preserve the observational and non-interventional nature of the study, the patients were approached for participation in the study after they were prescribed an add-on OAD. Recruitment was performed by a study group that worked independently of the treating physicians. The China PDS enrolled T2DM patients who were prescribed vildagliptin plus other OADs or another combination of dual OADs based on their treating physician’s decision. In the present study, only patients prescribed VM or AM were included in the subgroup analysis.
Data Collection
All data were recorded in an electronic case report form and collected in an electronic data capture system. The baseline characteristics included age, gender, BMI, baseline HbA1c, smoking history, drinking history, physical exercise, family history, duration of disease, and comorbidities. Patients were followed up at 3, 6 and 12 months after the initiation of combination therapy.
Endpoints
The composite primary endpoint was treatment success, defined as HbA1c < 7% after 12 months treatment and without any tolerability event, including hypoglycemia (symptoms suggestive of hypoglycemia, confirmed by self-monitored blood glucose < 3.1 mmol/L, and relief of symptoms after consumption of carbohydrates [19]), clinically significant weight gain (weight gain ≥ 3%, according to National Institutes of Health guidelines [20, 21]), and gastrointestinal events leading to discontinuation of the treatment (including decreased or increased appetite, abdominal pain, diarrhea, nausea, vomiting, dry mouth, dyspepsia, and weight loss [21]). The secondary endpoints were the glycemic control rate (HbA1c < 7%), change in HbA1c from baseline to 3, 6, 12 months, tolerability events, and adverse events (AEs). AEs were recorded using the Medical Dictionary for Regulatory Activities (MedDRA) version 15.1 (https://www.meddra.org/). Serious AEs (SAEs) were defined as follows: (1) fatal or life-threatening AEs; (2) persistent or severe disability; (3) requiring hospitalization or prolonging hospital stay due to the exacerbation of T2DM; and (4) requiring intervention to avoid AEs.
Statistical Analysis
The full-analysis set (FAS) included patients on VM or AM treatment and with at least one evaluation of effectiveness. The safety analysis set (SAS) included all patients who participated in this study with at least one safety evaluation.
The two groups were matched based on BMI and baseline HbA1c using propensity score matching (PSM). Continuous variables were expressed as the median with the inter-quartile range or as the mean ± the standard deviation according to whether or not they showed an accordance with a normal distribution, and they were compared using the Mann–Whitney U test or Student t test. Categorical variables were expressed as the frequency with the percentage and compared using the Chi-square test or Fisher exact test. Odds ratios (ORs) and relative risks (RRs) and 95% confidential intervals (CIs) were calculated. ORs for the composite primary endpoint were adjusted for the baseline variables recorded. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NY, USA). A two-sided P value of < 0.05 was considered to be statistically significant.
Results
Baseline Characteristics
Between June 2013 and April 2017, a total of 1657 patients were enrolled in the PDS. The detailed OAD treatment regimen prescribed to patients in the PDS are provided in ESM Table 2. In the present study, the SAS included 724 patients in the VM group and 185 in the AM group; the FAS included 604 patients in the VM group and 159 in the AM group. After PSM, there were 157 (26.0%) patients in the VM group and 157 (98.7%) in the AM group. The baseline characteristics of the study subjects before and after PSM are shown in Table 1. Before PSM, the patients in the VM group were younger than those in the AM group (median 52 vs. 58 years, P < 0.001), had a higher BMI (median 26.0 vs. 25.2 kg/m2, P = 0.003), had higher HbA1c levels (8.32% ± 1.05% vs. 7.84% ± 0.74%, P < 0.001), exercised less (92.4 vs. 96.9%, P = 0.045), and had a shorter duration of disease (median 34.2 vs. 52.4 months, P < 0.001). After PSM, there were no significant differences in baseline BMI (median 25.4 vs. 25.2 kg/m2) and HbA1c (7.85% ± 0.72% vs. 7.85 % ± 0.74%) (P > 0.05) between the VM group and AM group.
Composite Primary Endpoint
Both before and after PSM the success rates of the composite endpoint were higher in the VM group than in the AM group (Fig. 1), but the difference was not statistically significant (before PSM: 53.0 vs. 46.5%, P = 0.148; after PSM: 56.7 vs. 45.9%, P = 0.055). Compared with the AM group, the ORs for the primary endpoint in the VM group were 1.44 (95% CI 0.99–2.09) before PSM and 1.54 (95% CI 0.99–2.40) after PSM (Fig. 1).
Success rates of the composite primary endpoint in the vildagliptin add-on to metformin (VM) and α-glucosidase inhibitor add-on to metformin (AM) therapy groups. a Before propensity score matching (PSM), b after PSM. The composite primary endpoint was defined as glycemic control (hemoglobin A1c < 7%) at 12 months and without any tolerability event, including hypoglycemia, weight gain ≥ 3%, and gastrointestinal events leading to discontinuation of the treatment. CI Confidence interval, OR odds ratio
Glycemic Control
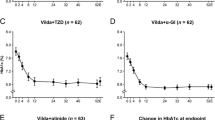
The glycemic control rate improved rapidly from baseline to 6 months and further improved at a slower rate after 6 months in both the VM and AM groups (Fig. 2a, b). Before PSM, the glycemic control rate was lower in the VM group at 3 months, but numerically higher at 6 and 12 months compared with the AM group, but the difference at each follow-up was not statistically significant (3 months: 40.5 vs. 44.7%; 6 months: 50.6 vs. 50.0%; 12 months: 54.0 vs. 50.9%; all P > 0.05). After PSM, the glycemic control rates were higher in the VM group than in the AM group at 3, 6 and 12 months, but the difference at each follow-up was not statistically significant (3 months: 48.1% vs. 43.9%; 6 months: 57.8% vs. 49.4%; 12 months: 58.6% vs. 50.3%; all P > 0.05). The reduction in HbA1c was more pronounced in the VM group than in the AM group before PSM, but with no significant difference at each follow-up (Fig. 2c; 3 months: − 1.13% ± 1.22% vs. − 0.75% ± 0.98%; 6 months: 1.24% ± 1.25% vs. − 0.71 % ± 1.17%; 12 months: − 1.27% ± 1.34% vs. − 0.75% ± 1.17%; all P > 0.05). After PSM, the reduction in HbA1c was similar in the two groups at 3 months and greater in the VM group at 6 and 12 months compared with the AM group, but with no significant difference at each follow-up (Fig. 2d; 3 months: − 0.75 ± 1.05% vs. − 0.75 ± 0.99%; 6 months: − 0.91 ± 0.95% vs. − 0.71 ± 1.17%; 12 months: − 0.94 ± 1.15% vs. − 0.75 ± 1.17%; all P > 0.05).
Glycemic control rates and mean changes of hemoglobin A1c (HbA1c) from baseline in the VM and AM groups. a Glycemic control rates (HbA1c < 7%) before propensity score matching (PSM) (inter-group comparison: 3 months, P = 0.358; 6 months, P = 0.893, 12 months, P = 0.496). b Glycemic control rates (HbA1c < 7%) after PSM (inter-group comparison: 3 months, P = 0.476; 6 months, P = 0.140, 12 months; P = 0.141). c Mean changes in HbA1c from baseline before PSM (inter-group comparison: 3 months, P = 0.931; 6 months, P = 0.094, 12 months; P = 0.202). Mean baseline HbA1c was 8.32% ± 1.05% in the VM group and 7.84% ± 0.74% in the AM group before PSM. d Mean changes of HbA1c from baseline after PSM (inter-group comparison: 3 months, P = 0.970; 6 months, P = 0.066, 12 months; P = 0.095). Mean baseline HbA1c was 7.85% ± 0.72% in the VM group and 7.85% ± 0.74% in the AM group after PSM. Error bars are the SD
Tolerability Events
Individual tolerability events are shown in Table 2. The results indicated that the VM group had lower risks of any tolerability event compared with the AM group (RR 0.53, 95% CI 0.33–0.83, P = 0.006). There were 46 (7.6%) and 20 (12.6%) patients with clinically significant weight gain (≥ 3%) in the VM and AM groups, respectively (RR 0.61, 95% CI 0.37–0.99, P = 0.048). No difference in hypoglycemia events was observed between the two groups (0.3 vs. 1.3%, P = 0.193). Two (1.3%) patients in the AM group experienced gastrointestinal events leading to discontinuation of the treatment in the AM group and none in the VM group (P = 0.043).
Adverse Events
Adverse events are shown in Table 3. The results indicated that the VM group (n = 724) had lower risks than the AM group (n = 185) of experiencing any AE (RR 0.64, 95% CI 0.41–1.00, P = 0.049) and any SAE (RR 0.45, 95% CI 0.25–0.81, P = 0.007). There were no significant between-group differences regarding AEs leading to discontinuation of the treatment (P = 0.687), hypoglycemic episodes (P = 0.140), or gastrointestinal events (P = 0.279). In the AM group, one patient discontinued the treatment due to a SAE (renal failure) and one patient died of cerebral hemorrhage.
Discussion
Real-world evidence is essential to verify the effects of DPP-4 inhibitors or AGIs as add-on therapy to metformin in Chinese patients with T2DM. The study reported here is the first real-world study that compares the glucose-lowering effect and tolerability of VM versus AM therapy in T2DM patients with inadequate glycemic control on monotherapy in China. The results suggest that vildagliptin as add-on to metformin monotherapy had a similar glucose-lowering effect as the AGI as add-on to metformin monotherapy, but with better safety.
In real-world clinical practice in China, AGI as add-on medication to metformin is the second most common non-vildagliptin dual OAD combination [18]. Pre-PSM baseline results from our study showed that in this real-life setting in China, compared to patients on VM, those on AM were older, had a lower BMI, a longer disease duration, and lower baseline HbA1c. These characteristics for patients on AM were in general agreement with those for patients on non-vildagliptin combination therapy in China [18] and are consistent with previous real-life data on acarbose use, with East Asian patients on acarbose reported to be older and less obese [22]. The distinct prescription pattern for VM versus that for other dual OAD, non-vildagliptin combination therapies (including AM) in China might be partially attributed to physician preference, which requires further research in the future.
When the lifestyle and genetic background of Asians are taken into account, both DPP-4 inhibitors and AGIs have been found to be suitable medications for Chinese patients with T2DM [5]. Previous RCTs have proven the efficacy and safety of AGIs or DPP-4 inhibitors as monotherapy as well as in combination with metformin [12,13,14,15,16, 23]. Gao et al. [12] reported that AGI monotherapy led to better glycemic control than the use of a DDP-4 inhibitor alone. Min et al. [13] demonstrated that the addition of a DPP-4 inhibitor to the treatment regimen of patients with T2DM inadequately controlled with an AGI could achieve better glycemic control without further increasing the risk of weight gain and hypoglycemia. Two meta-analyses have indicated that DPP-4 inhibitors could be considered as an add-on therapy to metformin in patients with inadequately controlled T2DM [15, 16]. In Japanese patients, Yokoh et al. [24] showed that the addition of sitagliptin to metformin or pioglitazone monotherapy showed better efficacy and tolerability than the addition of an AGI. In the present real-world study, the success rate of the composite primary endpoint and the glycemic control rate were higher in the VM group than in the AM group, and the reduction in HbA1c was more pronounced, although the differences were not statistically significant. A previous RCT showed the non-inferiority of vildagliptin versus acarbose monotherapy in terms of glycemic control efficacy [25]. Our study also showed a similar efficacy of vildagliptin and AGIs as add-ons to metformin in Chinese patients with T2DM in a real-world setting.
In the present study, we observed a trend to an increased frequency of gastrointestinal events for the AM group versus the VM group (3.2 vs. 1.9%). In addition, gastrointestinal events leading to discontinuation of the treatment occurred in two patients in the AM group, while none occurredin the VM group; this difference was statistically significant. Gastrointestinal events are common AEs in patients treated with AGIs [5]. A meta-analysis of RCTs showed that the incidence of gastrointestinal events increased in patients receiving AGIs as compared to those with placebo, but not in those receiving vildagliptin [26]. Further, a previous RCT that directly compared vildagliptin with an AGI as monotherapy [25] showed that the incidence of gastrointestinal events was decreased by 62% with vildagliptin. A network meta-analysis of RCTs also supported these findings, reporting that the incidence of gastrointestinal events after treatment with vildagliptin was lower than that after treatment with AGIs (OR 0.38; 95% CI 0.21–0.67) [26]. Notably, a relatively low incidence of gastrointestinal events was reported in the AM group in the present study, as compared with previous RCTs. One possible explanation is that the doses of AGI used in real-life are lower than those used in RCTs and that AGI-related AEs are dose-dependent [27]. Indeed, one large-scale study on acarbose showed that the highest dose of acarbose for most patients with T2DM was 50 mg three times daily in real-life, whereas the usual doses used in RCTs were ≥ 100 mg three times daily [28]. The inadequate up-titration of AGIs may be partially due to concerns for side effects. In addition, AGI-related gastrointestinal events have been reported to be less frequent in Asians than in non-Asians [12]. Alternatively, monitoring AEs in a real-world setting might be more difficult than in the context of a rigorous RCT and some under-reporting might have occurred in our study, especially for common mild AEs.
The occurrence of SAEs was significantly lower in the VM group than in the AM group, suggesting that VM therapy may have some specific advantages in terms of safety. For patients with severe comorbidities, vildagliptin may be a better choice than AGIs. Nevertheless, precaution needs to be taken regarding the interpretation of safety data from our study as the age/disease course after PSM were still unbalanced. In addition, as for the gastrointestinal events, SAEs might have suffered from underreporting if they were managed at another hospital than the one participating in the PDS and if the participant failed to report it during a study visit.
This study presents evidence with unselected patients that reflects the real-world setting. Baseline characteristics of the patient groups before PSM indicated that in real-world clinical practice, physicians in China may consider vildagliptin to be the preferred agent as add-on in combination with metformin in patients with higher baseline HbA1c, compared to AGIs. Notably, the glucose-lowering extent of DPP-4 inhibitors is related to the baseline HbA1c level (i.e., the higher the baseline HbA1c level, the more it will be reduced by DPP-4 inhibitors) [5]. In addition, the use of DPP-4 inhibitors does not increase the risk of hypoglycemia. Taken together, these factors may lead physicians in China to prefer vildagliptin over AGIs as an add-on combination with metformin.
Our study has several limitations. First, due to the non-randomization nature of the study, our results are subject to bias introduced by potential confounding factors. In real-world China, prescribers of vildagliptin are younger and have a shorter disease duration. To reserve a sufficient sample size for analysis, no matching for age and disease duration for the VM and AM groups was performed. Second, a short follow-up period and endpoints, such as the occurrence of cardiovascular events, were not analyzed.
Conclusion
In conclusion, this real-world study suggests that vildagliptin as add-on to metformin had a similar glucose-lowering effect as AGI as add-on to metformin, but with better safety.
References
Hu C, Jia W. Diabetes in China: epidemiology and genetic risk factors and their clinical utility in personalized medication. Diabetes. 2018;67:3–11.
Ji L, Hu D, Pan C, et al. Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 diabetes patients. Am J Med. 2013;126(925):e11–22.
American Diabetes Association. Standards of medical care in diabetes—2018 abridged for primary care providers. Clin Diabetes. 2018;36:14–37.
Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2018 executive summary. Endocr Pract. 2018;24:91–120.
Weng J, Ji L, Jia W, et al. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32:442–58.
Du YF, Ou HY, Beverly EA, Chiu CJ. Achieving glycemic control in elderly patients with type 2 diabetes: a critical comparison of current options. Clin Interv Aging. 2014;9:1963–80.
Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann NY Acad Sci. 2013;1281:64–91.
Kataoka M, Venn BJ, Williams SM, Te Morenga LA, Heemels IM, Mann JI. Glycaemic responses to glucose and rice in people of Chinese and European ethnicity. Diabet Med. 2013;30:e101–7.
Pathak R, Bridgeman MB. Dipeptidyl peptidase-4 (DPP-4) inhibitors in the management of diabetes. Pharm Therap. 2010;35:509–13.
Esposito K, Chiodini P, Maiorino MI, et al. A nomogram to estimate the HbA1c response to different DPP-4 inhibitors in type 2 diabetes: a systematic review and meta-analysis of 98 trials with 24,163 patients. BMJ Open. 2015;5:e005892.
Cai X, Han X, Luo Y, Ji L. Efficacy of dipeptidyl-peptidase-4 inhibitors and impact on beta-cell function in Asian and Caucasian type 2 diabetes mellitus patients: a meta-analysis. J Diabetes. 2015;7:347–59.
Gao X, Cai X, Yang W, Chen Y, Han X, Ji L. Meta-analysis and critical review on the efficacy and safety of alpha-glucosidase inhibitors in Asian and non-Asian populations. J Diabetes Investig. 2018;9:321–31.
Min SH, Yoon JH, Hahn S, Cho YM. Efficacy and safety of combination therapy with an alpha-glucosidase inhibitor and a dipeptidyl peptidase-4 inhibitor in patients with type 2 diabetes mellitus: a systematic review with meta-analysis. J Diabetes Investig. 2018;9:893–902.
Kaneko M, Narukawa M. Meta-analysis of dipeptidyl peptidase-4 inhibitors use and cardiovascular risk in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2016;116:171–82.
Foroutan N, Muratov S, Levine M. Safety and efficacy of dipeptidyl peptidase-4 inhibitors vs sulfonylurea in metformin-based combination therapy for type 2 diabetes mellitus: systematic review and meta-analysis. Clin Invest Med. 2016;39:E48–62.
Mishriky BM, Cummings DM, Tanenberg RJ. The efficacy and safety of DPP4 inhibitors compared to sulfonylureas as add-on therapy to metformin in patients with Type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2015;109:378–88.
Mathieu C, Degrande E. Vildagliptin: a new oral treatment for type 2 diabetes mellitus. Vasc Health Risk Manag. 2008;4:1349–60.
Zang L, Han Y, Chen L, et al. Comparison of the effectiveness and safety of vildagliptin add-on to metformin versus other oral dual antidiabetes agents in patients with type 2 diabetes: the China Prospective Diabetes Study. Diabetes Ther. 2019;10:1391–405.
Pan C, Xing X, Han P, et al. Efficacy and tolerability of vildagliptin as add-on therapy to metformin in Chinese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:737–44.
National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Publication No. 98-4083. Bethesda: National Institutes of Health; National Heart, Lung, and Blood Institute; 1998.
Holmskov M, Storebo OJ, Moreira-Maia CR, et al. Gastrointestinal adverse events during methylphenidate treatment of children and adolescents with attention deficit hyperactivity disorder: a systematic review with meta-analysis and trial sequential analysis of randomised clinical trials. PLoS One. 2017;12:e0178187.
Schnell O, Weng J, Sheu WH, et al. Acarbose reduces body weight irrespective of glycemic control in patients with diabetes: results of a worldwide, non-interventional, observational study data pool. J Diabetes Complicat. 2016;30:628–37.
Iwamoto Y, Kashiwagi A, Yamada N, et al. Efficacy and safety of vildagliptin and voglibose in Japanese patients with type 2 diabetes: a 12-week, randomized, double-blind, active-controlled study. Diabetes Obes Metab. 2010;12:700–8.
Yokoh H, Kobayashi K, Sato Y, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin compared with alpha-glucosidase inhibitor in Japanese patients with type 2 diabetes inadequately controlled on metformin or pioglitazone alone (Study for an Ultimate Combination Therapy to Control Diabetes with Sitagliptin-1): a multicenter, randomized, open-label, non-inferiority trial. J Diabetes Investig. 2015;6:182–91.
Pan C, Yang W, Barona JP, et al. Comparison of vildagliptin and acarbose monotherapy in patients with Type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med. 2008;25:435–41.
Wu S, Chai S, Yang J, et al. Gastrointestinal adverse events of dipeptidyl peptidase 4 inhibitors in type 2 diabetes: a systematic review and network meta-analysis. Clin Ther. 2017;39(1780–89):e33.
van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. Alpha-glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154–63.
Weng J, Soegondo S, Schnell O, et al. Efficacy of acarbose in different geographical regions of the world: analysis of a real-life database. Diabetes Metab Res Rev. 2015;31:155–67.
Acknowledgements
The authors acknowledge all the patients and physicians participating in this study. We acknowledge the ethics committee of Chinese PLA General Hospital for piloting the ethical review of this study.
Funding
This study and the Rapid Service Fee were funded by Novartis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Yulong Chen, Quanmin Li, Ying Han, Hongmei Ji, Mingjun Gu, Rongwen Bian, Weiguang Ding, and Yiming Mu have nothing to disclose. Jian Cheng is an employee of Novartis China.
Compliance with Ethics Guidelines
The study was conducted in compliance with the Declaration of Helsinki of 1964 and its later amendments. The study protocol was independently approved by the ethics committee/institutional review board of Chinese PLA General Hospital (central committee) and each participating study center (ESM Table 1). Written informed consent for the study was obtained from all participants before enrollment. Since no individual person’s data were included in this manuscript, the consent for publication was waived by the ethics committees.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11280098.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Chen, Y., Li, Q., Han, Y. et al. Vildagliptin Versus α-Glucosidase Inhibitor as Add-On to Metformin for Type 2 Diabetes: Subgroup Analysis of the China Prospective Diabetes Study. Diabetes Ther 11, 247–257 (2020). https://doi.org/10.1007/s13300-019-00742-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13300-019-00742-8