Abstract
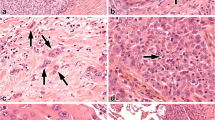
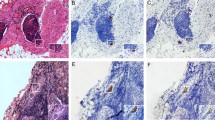
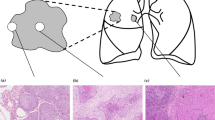
Recurrences occur in 30 % of lung cancer patients after radical therapy; however, known prognostic factors are not always effective. In this study, we investigated whether the frequency of squamous non-small cell lung cancer (NSCLC) recurrence depends on the presence of reactive lesions in tumor-adjacent bronchial epithelium. Specimens of adjacent lung tissue from 104 patients with squamous NSCLC were used for the determination of basal cell hyperplasia (BCH) and squamous metaplasia (SM) and for the analysis of the expression of Ki-67, p53, Bcl-2, and CD138. We found that recurrence was observed in 36.7 % of patients with BCH combined with SM (BCH + SM+) in the same bronchus, compared with 1.8 % in patients with isolated BCH (BCH + SM−; odds ratio (OR) 31.26, 95 % confidence interval (CI) 3.77–258.60; p = 0.00002). The percentage of Ki-67-positive cells was significantly higher in BCH + SM+ than in BCH + SM− (34.9 vs. 18.3 %; effect size 2.86, 95 % CI 2.23–3.47; p = 0.003). P53 expression was also more significant in BCH + SM+ than in BCH + SM− (14.4 vs. 9.6 %; effect size 1.22, 95 % CI 0.69–1.76; p = 0.0008). In contrast, CD138 expression was lower in BCH + SM+ than in BCH + SM− (21.8 vs. 38.5 %; effect size −6.26, 95 % CI −7.31 to −5.22; p = 0.003). Based on our results, we concluded that the co-presence of reactive bronchial lesions is associated with the development of recurrent squamous NSCLC and may be a negative prognostic indicator. In addition, significant differences in Ki-67, p53, and CD138 expression exist between isolated BCH and BCH combined with SM that probably reflect part of biological differences, which could relate to the mechanism of lung cancer recurrence.



Similar content being viewed by others
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors. New York, USA: Wiley-Blackwell; 2009.
Poleri C, Morero JL, Nieva B, Vazquez MF, Rodriguez C, de Titto E, et al. Risk of recurrence in patients with surgically resected stage I non-small cell lung carcinoma: histopathologic and immunohistochemical analysis. Chest. 2003;123(6):1858–67.
Lopez Guerra JL, Gomez DR, Lin SH, Levy LB, Zhuang Y, Komaki R, et al. Risk factors for local and regional recurrence in patients with resected N0-N1 non-small-cell lung cancer, with implications for patient selection for adjuvant radiation therapy. Ann Oncol. 2013;24(1):67–74.
Gold KA, Kim ES, Liu DD, Yuan P, Behrens C, Solis LM, et al. Prediction of survival in resected non-small cell lung cancer using a protein expression-based risk model: implications for personalized chemoprevention and therapy. Clin Cancer Res. 2014;20(7):1946–54.
O’Callaghan DS, O’Donnell D, O’Connell F, O’Byrne KJ. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. 2010;5(12):2024–36.
Kuo CH, Wu CY, Lee KY, Lin SM, Chung FT, Lo YL, et al. Chronic obstructive pulmonary disease in stage I non-small cell lung cancer that underwent anatomic resection: the role of a recurrence promoter. Copd. 2014;11(4):407–13.
Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–30.
Dacic S. Pulmonary preneoplasia. Arch Pathol Lab Med. 2008;132(7):1073–8.
Greenberg AK, Yee H, Rom WN. Preneoplastic lesions of the lung. Respir Res. 2002;3:20.
Ishizumi T, McWilliams A, MacAulay C, Gazdar A, Lam S. Natural history of bronchial preinvasive lesions. Cancer Metastasis Rev. 2010;29(1):5–14.
Kerr KM, Fraire AE. Preinvasive disease. In: Tomashefski JF, Cagle PT, Farver CF, Fraire AE, editors. Dail and Hammar’s pulmonary pathology. Springer: Science+Business Media; 2008. p. 158–206.
Kadara H, Wistuba II. Molecular biology of lung preneoplasia. In: Roth JA, Hong WK, Komaki RU, editors. Lung cancer. Fourth ed. Hoboken: Wiley; 2014. p. 110–28.
Mascaux C, Laes JF, Anthoine G, Haller A, Ninane V, Burny A, et al. Evolution of microRNA expression during human bronchial squamous carcinogenesis. Eur Respir J. 2009;33(2):352–9.
van Boerdonk RAA, Sutedja TG, Snijders PJF, Reinen E, Wilting SM, van de Wiel MA, et al. DNA copy number alterations in endobronchial squamous metaplastic lesions predict lung cancer. Am J Respir Crit Care Med. 2011;184(8):948–56.
Moskalev EA, Jandaghi P, Fallah M, Manoochehri M, Botla SK, Kolychev OV, et al. GHSR DNA hypermethylation is a common epigenetic alteration of high diagnostic value in a broad spectrum of cancers. Oncotarget. 2015;6(6):4418–27.
Bjaanaes MM, Halvorsen AR, Solberg S, Jorgensen L, Dragani TA, Galvan A, et al. Unique microRNA-profiles in EGFR-mutated lung adenocarcinomas. Int J Cancer. 2014;135(8):1812–21.
Wang GF, Lai MD, Yang RR, Chen PH, Su YY, Lv BJ, et al. Histological types and significance of bronchial epithelial dysplasia. Mod Pathol. 2006;19(3):429–37.
Pankova OV, Perelmuter VM, Savenkova OV. Characteristics of proliferation marker expression and apoptosis regulation depending on the character of disregenerator changes in bronchial epithelium of patients with squamous cell lung cancer. Siberian J Oncol. 2010;5:36–41.
Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, Yatabe Y, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–85.
Banerjee AK. Preinvasive lesions of the bronchus. J Thorac Oncol. 2009;4(4):545–51.
Chyczewski L, Niklinski J, Chyczewska E, Niklinska W, Naumnik W. Morphological aspects of carcinogenesis in the lung. Lung Cancer. 2001;34 Suppl 2:S17–25.
Kerr KM. Pulmonary preinvasive neoplasia. J Clin Pathol. 2001;54(4):257–71.
Kerr KM, Popper HH. The differential diagnosis of pulmonary pre-invasive lesions. Eur Respir Mon. 2007;39:37–62.
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. World Health Organization classification of tumors: pathology and genetics of tumors of the lung, pleura, thymus and heart. 4th ed. Lyon, France: IARC Press; 2004.
Pankova OV, Perelmuter VM, Tuzikov SA, Savenkova OV. Effect of neoadjuvant chemotherapy on the spectrum and expression profile of disregeneratory changes in the bronchial mucosa in patients with non-small cell lung cancer. Siberian J Oncol. 2012;3:79–83.
Melamed MR. Lung cancer screening results in the National Cancer Institute New York study. Cancer. 2000;89 Suppl 11:2356–62.
Bota S, Auliac JB, Paris C, Metayer J, Sesboue R, Nouvet G, et al. Follow-up of bronchial precancerous lesions and carcinoma in situ using fluorescence endoscopy. Am J Respir Crit Care Med. 2001;164(9):1688–93.
Breuer RH, Pasic A, Smit EF, van Vliet E, Vonk Noordegraaf A, Risse EJ, et al. The natural course of preneoplastic lesions in bronchial epithelium. Clin Cancer Res. 2005;11(2 Pt 1):537–43.
Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5:75.
Hattar K, Franz K, Ludwig M, Sibelius U, Wilhelm J, Lohmeyer J, et al. Interactions between neutrophils and non-small cell lung cancer cells: enhancement of tumor proliferation and inflammatory mediator synthesis. Cancer Immunol Immunother. 2014;63(12):1297–306.
Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–31.
Pankova ОV, Perelmuter VМ, Savenkova ОV, Denisov ЕV, Vasilyev SА, Skryabin NА, et al. Characteristics of bronchial mucosal inflammatory response in sites of basal cell hyperplasia and squamous metaplasia combined with squamous cell lung cancer. Siberian J Oncol. 2012;5:28–33.
Gharbaran R. Advances in the molecular functions of syndecan-1 (SDC1/CD138) in the pathogenesis of malignancies. Crit Rev Oncol Hematol. 2014;94(1):1–17.
Shah L, Walter KL, Borczuk AC, Kawut SM, Sonett JR, Gorenstein LA, et al. Expression of syndecan-1 and expression of epidermal growth factor receptor are associated with survival in patients with nonsmall cell lung carcinoma. Cancer. 2004;101(7):1632–8.
Acknowledgments
The study was supported by the Russian Scientific Foundation (project #14-15-00350). E.V. Denisov was supported by Tomsk State University Competitiveness Improvement Program.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 192 kb)
Rights and permissions
About this article
Cite this article
Pankova, O.V., Denisov, E.V., Ponomaryova, A.A. et al. Recurrence of squamous cell lung carcinoma is associated with the co-presence of reactive lesions in tumor-adjacent bronchial epithelium. Tumor Biol. 37, 3599–3607 (2016). https://doi.org/10.1007/s13277-015-4196-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4196-2