Abstract
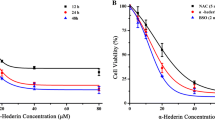
Esophageal cancer is of high prevalence and poor prognosis. Hesperetin has been reported to exert antitumor ability by inducing apoptosis in many cancers in vitro and in vivo without obvious toxicity. However, there is no study concerning about the effect of hesperetin on esophageal cancer. In this study, we aimed to investigate whether hesperetin could induce apoptosis in esophageal cancer cells and explore its potential mechanism. We found that hesperetin induced esophageal cancer cells apoptosis in a concentration-dependent and time-dependent manner compared with the untreated cells. Hoechst 33258 staining and flow cytometry analysis showed more apoptotic cells in the hesperetin-treated group (p < 0.05, respectively). The intracellular reactive oxygen species (ROS) increased significantly, and glutathione (GSH) was depleted. The loss of △Ψ m was more tremendous in the hesperetin-treated cells. N-acetylcysteine (NAC) reduced the proapoptotic ability of hesperetin, while DL-buthionine-S, R-sulfoximine (BSO) enhanced the anticancer effect. Western blotting showed that the expression levels of cytochrome C (Cyt C) and apoptosis-inducing factor (AIF) decreased in mitochondria and increased in cytoplasm (p < 0.05). The levels of intracellular cleaved caspase-9, cleaved caspase-3, Apaf-1, Bcl-2-associated X protein (Bax), and suppressor of fused (SuFu) increased, while B cell lymphoma 2 (Bcl-2) and Survivin decreased. What is more, in xenograft tumor model, hesperetin inhibited the tumor growth significantly via induction of cell apoptosis which was detected by TUNEL assay (p < 0.05). Taken together, our study demonstrated that hesperetin could induce cell apoptosis in esophageal cancer cells via mitochondrial-mediated intrinsic pathway by accumulation of ROS.




Similar content being viewed by others
References
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Shahbaz Sarwar CM, Luketich JD, Landreneau RJ, et al. Esophageal cancer: an update. Int J Surg. 2010;8(6):417–22.
Choi EJ. Hesperetin induced G1-phase cell cycle arrest in human breast cancer MCF-7 cells: involvement of CDK4 and p21. Nutr Cancer. 2007;59(1):115–9.
Sambantham S, Radha M, Paramasivam A, et al. Molecular mechanism underlying hesperetin-induced apoptosis by in silico analysis and in prostate cancer PC-3 cells. Asian Pac J Cancer Prev. 2013;14(7):4347–52.
Sivagami G, Vinothkumar R, Preethy CP, et al. Role of hesperetin (a natural flavonoid) and its analogue on apoptosis in HT-29 human colon adenocarcinoma cell line—a comparative study. Food Chem Toxicol. 2012;50(3–4):660–71.
Zhang J, Song J, Wu D, et al. Hesperetin induces the apoptosis of hepatocellular carcinoma cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species, ATP and calcium. Med Oncol. 2015;32(4):101.
Palit S, Kar S, Sharma G, et al. Hesperetin induces apoptosis in breast carcinoma by triggering accumulation of ROS and activation of ASK1/JNK pathway. J Cell Physiol. 2014.
Alshatwi AA, Ramesh E, Periasamy VS, et al. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fundam Clin Pharmacol. 2013;27(6):581–92.
Roohbakhsh A, Parhiz H, Soltani F, et al. Molecular mechanisms behind the biological effects of hesperidin and hesperetin in the prevention of cancer and cardiovascular diseases. Life Sci. 2015;124:64–74.
Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48(2):158–67.
DeNicola GM, Karreth FA, Humpton TJ, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–9.
Satoh H, Moriguchi T, Takai J, Ebina M, Yamamoto M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013;73(13):4158–68.
Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci. 2000;899(1):136–47.
Lee HH, Park C, Jeong JW, et al. Apoptosis induction of human prostate carcinoma cells by cordycepin through reactive oxygen species-mediated mitochondrial death pathway. Int J Oncol. 2013;42(3):1036–44.
Ryter SW, Kim HP, Hoetzel A, et al. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9(1):49–89.
Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11(9):621–32.
Kim HE, Du F, Fang M, et al. Formation of apoptosome is initiated by cytochrome c-induced dATP hydrolysis and subsequent nucleotide exchange on Apaf-1. Proc Natl Acad Sci U S A. 2005;102(49):17545–50.
He J, Shao K. The epidemiology, current status of management, challenge and future strategy for esophageal cancer in China. China Oncol. 2011;21(7):501–4.
Weidner C, Rousseau M, Plauth A, et al. Melissa officinalis extract induces apoptosis and inhibits proliferation in colon cancer cells through formation of reactive oxygen species. Phytomedicine. 2015;22(2):262–70.
Lin B, Tan X, Liang J, et al. A reduction in reactive oxygen species contributes to dihydromyricetin-induced apoptosis in human hepatocellular carcinoma cells. Sci Rep. 2014;4:7041.
Dickens LS, Powley IR, Hughes MA, MacFarlane M. The ‘complexities’ of life and death: death receptor signalling platforms. Exp Cell Res. 2012;318(11):1269–77.
Mohamed H, Hidemichi W, Ali A, et al. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845.
Roohbakhsh A, Parhiz H, Soltani F, et al. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin—a mini-review. Life Sci. 2014;113(1–2):1–6.
Parhiz H, Roohbakhsh A, Soltani F, et al. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 2015;29(3):323–31.
Ouyang L, Shi Z, Zhao S, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487–98.
Lopez J, Tait SW. Mitochondrial apoptosis: killing cancer using the enemy within. Br J Cancer. 2015;112(6):957–62.
Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–41.
Norishi U. Ceramide-induced apoptosis in renal tubular cells: a role of mitochondria and sphingosine-1-phosphate. Int J Mol Sci. 2015;16(3):5076–124.
Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12(3):329–41.
Franklin JL. Redox regulation of the intrinsic pathway in neuronal apoptosis. Antioxid Redox Signal. 2011;14(8):1437–48.
Acknowledgments
The study was supported by the research grants from the Natural Science Foundation of Hubei Province (No. 2014CKB494).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal assays were approved by the ethics committee for animal research of Wuhan University, China. All experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Wu, D., Zhang, J., Wang, J. et al. Hesperetin induces apoptosis of esophageal cancer cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species. Tumor Biol. 37, 3451–3459 (2016). https://doi.org/10.1007/s13277-015-4176-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4176-6