Abstract
Background
Obesity, which is defined as the excess accumulation of body fat, poses metabolic diseases that result in significant health risks. Since conventional anti-obesity medications are known to have significant side effects, we tried a pharmacological approach with a natural product. Ginseng (Panax ginseng) is a traditional Asian medicine that possesses antioxidant, anti-inflammatory, and anti-obesogenic properties. However, the mechanism of the anti-obesity effects of ginseng leaf extract (GLE) is not yet understood.
Objective
We investigated the mechanism by which GLE inhibits the differentiation of 3T3-L1 preadipocytes.
Results
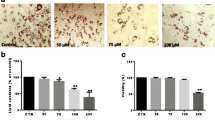
GLE treatment was administered throughout the 8 days differentiation period or at three stages of adipocyte differentiation (early: days 0–2; intermediate: days 2–4; or late: after day 4). During adipocyte differentiation, GLE treatment significantly inhibited 3T3-L1 preadipocyte differentiation at the early stage, leading to a notable reduction in lipid accumulation and a decrease in the expression of crucial adipogenic transcription factors that regulate adipocyte differentiation. GLE also increased the expression of HO-1 and Wnt/β-catenin signaling in a dose-dependent manner during adipocyte differentiation. To evaluate the role of HO-1 induced by GLE, we used HO-1 inhibitor SnPP and HO-1 siRNA. Attenuation of HO-1 function and expression inhibited the decrease in lipid accumulation and adipogenic transcription factor expression caused by GLE; furthermore, inhibition of HO-1 suppressed Wnt/β-catenin signaling.
Conclusions
Overall, our results suggest that GLE inhibits the differentiation of 3T3-L1 preadipocytes by regulating HO-1 expression and Wnt/β-catenin signaling. Therefore, GLE could have preventive uses as a natural product for the treatment of obesity.





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bluher M (2020) Metabolically healthy obesity. Endocr Rev. https://doi.org/10.1210/endrev/bnaa004
Cao Y (2010) Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov 9(2):107–115
Chang E, Kim CY (2019) Natural products and obesity: a focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules. https://doi.org/10.3390/molecules24061157
Christodoulides C, Lagathu C, Sethi JK, Vidal-Puig A (2009) Adipogenesis and WNT signalling. Trends Endocrinol Metab 20(1):16–24
Dai S et al (2018) Ginsenoside Rb2 promotes glucose metabolism and attenuates fat accumulation via AKT-dependent mechanisms. Biomed Pharmacother 100:93–100
de Winter TJJ, Nusse R (2021) Running against the Wnt: how Wnt/beta-catenin suppresses adipogenesis. Front Cell Dev Biol 9:627429
Farmer SR (2006) Transcriptional control of adipocyte formation. Cell Metab 4(4):263–273
Gao Y et al (2020) Ginsenoside Rg1 protects mice against streptozotocin-induced type 1 diabetic by modulating the NLRP3 and Keap1/Nrf2/HO-1 pathways. Eur J Pharmacol 866:172801
Gregoire FM, Smas CM, Sul HS (1998) Understanding adipocyte differentiation. Physiol Rev 78(3):783–809
Harms M, Seale P (2013) Brown and beige fat: development, function and therapeutic potential. Nat Med 19(10):1252–1263
Hur J et al (2021) Ginseng leaf extract ameliorates the survival of endotoxemic mice by inhibiting the release of high mobility group box 1. J Food Biochem 45(7):e13805
Khitan Z, Harsh M, Sodhi K, Shapiro JI, Abraham NG (2014) HO-1 upregulation attenuates adipocyte dysfunction, obesity, and isoprostane levels in mice fed high fructose diets. J Nutr Metab 2014:980547
Kim CY, Kang B, Suh HJ, Choi HS (2018) Red ginseng-derived saponin fraction suppresses the obesity-induced inflammatory responses via Nrf2-HO-1 pathway in adipocyte-macrophage co-culture system. Biomed Pharmacother 108:1507–1516
Koh EJ et al (2017) Ginsenoside Rg1 suppresses early stage of adipocyte development via activation of C/EBP homologous protein-10 in 3T3-L1 and attenuates fat accumulation in high fat diet-induced obese zebrafish. J Ginseng Res 41(1):23–30
Laudes M (2011) Role of WNT signalling in the determination of human mesenchymal stem cells into preadipocytes. J Mol Endocrinol 46(2):R65-72
Lee SH et al (2011) Persicaria hydropiper (L.) spach and its flavonoid components, isoquercitrin and isorhamnetin, activate the Wnt/beta-catenin pathway and inhibit adipocyte differentiation of 3T3-L1 cells. Phytother Res 25(11):1629–1635
Lee HJ, Lee HS, Cho HJ, Kim SY, Suh HJ (2012a) Utilization of hydrolytic enzymes for the extraction of ginsenosides from Korean ginseng leaves. Process Biochem 47(3):538–543
Lee OH, Seo MJ, Choi HS, Lee BY (2012b) Pycnogenol(R) inhibits lipid accumulation in 3T3-L1 adipocytes with the modulation of reactive oxygen species (ROS) production associated with antioxidant enzyme responses. Phytother Res 26(3):403–411
Lee SG, Lee YJ, Jang MH, Kwon TR, Nam JO (2017) Panax ginseng leaf extracts exert anti-obesity effects in high-fat diet-induced obese rats. Nutrients. https://doi.org/10.3390/nu9090999
Lee JW et al (2017) Comprehensive profiling and quantification of ginsenosides in the root, stem, leaf, and berry of Panax ginseng by UPLC-QTOF/MS. Molecules. https://doi.org/10.3390/molecules22122147
Li Y, Zhang W (2022) Effect of ginsenoside Rb2 on a myocardial cell model of coronary heart disease through Nrf2/HO-1 signaling pathway. Biol Pharm Bull 45(1):71–76
Li JB, Zhang R, Han X, Piao CL (2018) Ginsenoside Rg1 inhibits dietary-induced obesity and improves obesity-related glucose metabolic disorders. Braz J Med Biol Res 51(4):e7139
Li J et al (2021) Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. J Transl Med 19(1):96
Liu H et al (2018) Antiobesity effects of ginsenoside Rg1 on 3T3-L1 preadipocytes and high fat diet-induced obese mice mediated by AMPK. Nutrients. https://doi.org/10.3390/nu10070830
Liu H et al (2019) Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food Funct 10(6):3603–3614
Lu JM, Yao Q, Chen C (2009) Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol 7(3):293–302
Oh J et al (2012) Ginseng and its active components ginsenosides inhibit adipogenesis in 3T3-L1 cells by regulating MMP-2 and MMP-9. Evid Based Complement Alternat Med 2012:265023
Olshansky SJ et al (2005) A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 352(11):1138–1145
Prestwich TC, Macdougald OA (2007) Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol 19(6):612–617
Rahman N, Jeon M, Kim YS (2016) Delphinidin, a major anthocyanin, inhibits 3T3-L1 pre-adipocyte differentiation through activation of Wnt/beta-catenin signaling. BioFactors 42(1):49–59
Ross SE et al (2000) Inhibition of adipogenesis by Wnt signaling. Science 289(5481):950–953
Shan Y et al (2022) Ginsenoside Rg3 ameliorates acute pancreatitis by activating the NRF2/HO-1-mediated ferroptosis pathway. Int J Mol Med. https://doi.org/10.3892/ijmm.2022.5144
Shi W, Wang YT, Li J, Zhang HQ, Ding L (2007) Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem 102(3):664–668
Shin SS, Yoon M (2018) Korean red ginseng (Panax ginseng) inhibits obesity and improves lipid metabolism in high fat diet-fed castrated mice. J Ethnopharmacol 210:80–87
Shin JE, Jeon SH, Lee SJ, Choung SY (2022) The administration of Panax ginseng berry extract attenuates high-fat-diet-induced sarcopenic obesity in C57BL/6 mice. Nutrients. https://doi.org/10.3390/nu14091747
Siraj FM, SathishKumar N, Kim YJ, Kim SY, Yang DC (2015) Ginsenoside F2 possesses anti-obesity activity via binding with PPARgamma and inhibiting adipocyte differentiation in the 3T3-L1 cell line. J Enzyme Inhib Med Chem 30(1):9–14
Tian L et al (2017) Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis 8(1):e2559
Tsai YC et al (2017) Heme oxygenase-1 mediates anti-adipogenesis effect of raspberry ketone in 3T3-L1 cells. Phytomedicine 31:11–17
Tsai YC et al (2020) Involvement of the p62/Nrf2/HO-1 pathway in the browning effect of irisin in 3T3-L1 adipocytes. Mol Cell Endocrinol 514:110915
Vanella L et al (2013) Increased heme-oxygenase 1 expression in mesenchymal stem cell-derived adipocytes decreases differentiation and lipid accumulation via upregulation of the canonical Wnt signaling cascade. Stem Cell Res Ther 4(2):28
Wang CZ, Wu JA, McEntee E, Yuan CS (2006) Saponins composition in American ginseng leaf and berry assayed by high-performance liquid chromatography. J Agric Food Chem 54(6):2261–2266
Yan X et al (2022) Ginseng oligosaccharides protect neurons from glutamate-induced oxidative damage through the Nrf2/HO-1 signaling pathway. Food Funct 13(16):8605–8615
Yang JW, Kim SS (2015) Ginsenoside Rc promotes anti-adipogenic activity on 3T3-L1 adipocytes by down-regulating C/EBPalpha and PPARgamma. Molecules 20(1):1293–1303
Yang SO et al (2014) Classification of ginseng berry (Panax ginseng C.A. MEYER) extract using 1H NMR spectroscopy and its inhibition of lipid accumulation in 3 T3–L1 cells. BMC Complement Altern Med. https://doi.org/10.1186/1472-6882-14-455
Yu X et al (2015) Ginsenoside Rb1 ameliorates liver fat accumulation by upregulating perilipin expression in adipose tissue of db/db obese mice. J Ginseng Res 39(3):199–205
Zeng X, Li J, Li Z (2015) Ginsenoside Rd mitigates myocardial ischemia-reperfusion injury via Nrf2/HO-1 signaling pathway. Int J Clin Exp Med 8(8):14497–14504
Zhang L, Virgous C, Si H (2017) Ginseng and obesity: observations and understanding in cultured cells, animals and humans. J Nutr Biochem 44:1–10
Zhang Z et al (2022) Ginsenoside Rg1 inhibits oxidative stress and inflammation in rats with spinal cord injury via Nrf2/HO-1 signaling pathway. NeuroReport 33(2):81–89
Zheng Y et al (2020) Preclinical research on a mixture of red ginseng and licorice extracts in the treatment and prevention of obesity. Nutrients. https://doi.org/10.3390/nu12092744
Zhou J et al (2021) Ginsenoside F2 suppresses adipogenesis in 3T3-L1 cells and obesity in mice via the AMPK pathway. J Agric Food Chem 69(32):9299–9312
Acknowledgements
We would like to appreciate bioedit [www.bioedit.kr] for English language editing.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2021R1A2C1007526).
Author information
Authors and Affiliations
Contributions
HGL, JH and HGS contributed to conceptualization and design. HGL and JH conducted methodology and investigation. JH and JPW validated the results. HGL visualized all data and drafted the original manuscript. HGS managed and supervised the entire study and handled manuscript review and editing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Hyuk Gyoon Lee declares that he has no conflict of interest. Jinwoo Hur declares that he has no conflict of interest. Jun Pil Won declares that he has no conflict of interest. Han Geuk Seo declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, H.G., Hur, J., Won, J.P. et al. Heme oxygenase-1 mediates the inhibitory effect of ginseng (Panax ginseng) leaf extract on differentiation in 3T3-L1 adipocytes. Mol. Cell. Toxicol. (2023). https://doi.org/10.1007/s13273-023-00408-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s13273-023-00408-4