Abstract
Background
Radiation damage and the cellular response has been studied in various direction, however, the synergic effects of radiation damage with environmental pollution on cells or tissues remained poorly understood. In particular, gene and pathway regulation by low-dose radiation exposure remains unclear. Dust and air pollution in Asian countries contains metal oxide and titanium dioxide nanoparticles (TiO2NPs), which exacerbate respiratory distress.
Objective
To explore the synergic injury of radiation damage with air pollution, we examined the effects of low-dose-rate radiation with TiO2NPs on pulmonary response in mice.
Results
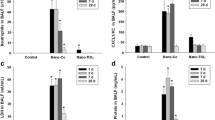
Thirty-six mice (C57bl/6) were divided into six groups: sham, 0.1 Gy, 0.3 Gy, TiO2NPs, TiO2NPs + 0.1 Gy, and TiO2NPs + 0.3 Gy group. Mice were irradiated at a low-dose-rate at a dose of 0.1 Gy (0.182 mGy/h) and 0.3 Gy (0.554 mGy/h) for 24 days and exposed to TiO2NPs by intranasal injection at a dose of 0.1 mg daily for 4 days (from day 21 to 24). The combination of low-dose-rate radiation and TiO2NPs caused significantly more pulmonary inflammation via MAPK phosphorylation in mice than did each stimulus alone.
Conclusion
We conclude that while exposure to each of these two distinct stimuli alone does not cause notable lung damage, they may potentially cause lung damage when combined owing to their synergistic effects. Therefore, we should pay attention to the possible combined effects of low-dose radiation and exposure to TiO2NPs, considering their potential danger in patients with respiratory problems.





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ali YF, Cucinotta FA, Ning-Ang L, Zhou G (2020) Cancer risk of low dose ionizing radiation. Front Phys 8:234
Atkinson JJ, Senior RM (2003) Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol 28:12–24
Baranowska-Wójcik E, Szwajgier D, Oleszczuk P, Winiarska-Mieczan A (2020) Effects of titanium dioxide nanoparticles exposure on human health—a review. Biol Trace Elem Res 193:118–129
Bennett B (2006) c-Jun N-terminal kinase-dependent mechanisms in respiratory disease. Eur Respir J 28:651–661
Chen Q et al (2018) TiO2 nanoparticles cause mitochondrial dysfunction, activate inflammatory responses, and attenuate phagocytosis in macrophages: a proteomic and metabolomic insight. Redox Biol 15:266–276
Corbel M, Belleguic C, Boichot E, Lagente V (2002) Involvement of gelatinases (MMP-2 and MMP-9) in the development of airway inflammation and pulmonary fibrosis. Cell Biol Toxicol 18:51–61
Dasari KB, Cho H, JaćimovićSunYim RG-MY-H (2020) Chemical composition of asian dust in daejeon, korea, during the spring season. ACS Earth Space Chem 4:1227–1236
Egbuna C et al (2021) Toxicity of nanoparticles in biomedical application: nanotoxicology. J Toxicol 2021:9954443
Eldh T et al (2012) Radiation-induced changes in breathing frequency and lung histology of C57BL/6J mice are time-and dose-dependent. Strahlenther Onkol 188:274–281
Eom H-J, Choi J (2009) Oxidative stress of CeO2 nanoparticles via p38-Nrf-2 signaling pathway in human bronchial epithelial cell, Beas-2B. Toxicol Lett 187:77–83
Fang W et al (2017) Modulation of mitogen-activated protein kinase attenuates sepsis-induced acute lung injury in acute respiratory distress syndrome rats. Mol Med Report 16:9652–9658
Feinendegen L (2005) Evidence for beneficial low level radiation effects and radiation hormesis. Br J Radiol 78:3–7
Geiser M, Kreyling WG (2010) Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol 7:1–17
Guha M et al (2001) Lipopolysaccharide activation of the MEK-ERK1/2 pathway in human monocytic cells mediates tissue factor and tumor necrosis factor alpha expression by inducing Elk-1 phosphorylation and Egr-1 expression. Blood 98:1429–1439
Hung L-Y et al (2019) Macrophages promote epithelial proliferation following infectious and non-infectious lung injury through a Trefoil factor 2-dependent mechanism. Mucosal Immunol 12:64–76
Jo WS et al (2022) Low dose rate radiation regulates M2-like macrophages in an allergic airway inflammation mouse model. Dose-Response 20:15593258221117348
Kang IG, Jung JH, Kim ST (2012) Asian sand dust enhances allergen-induced th2 allergic inflammatory changes and mucin production in BALB/c mouse lungs. Allergy Asthma Immunol Res 4:206–213
Khan I, Saeed K, Khan I (2019) Nanoparticles: properties, applications and toxicities. Arab J Chem 12:908–931
Kim JS et al (2015) Continuous exposure to low-dose-rate gamma irradiation reduces airway inflammation in ovalbumin-induced asthma. PLoS ONE 10:e0143403
Kim B-G, Lee P-H, Lee S-H, Park M-K, Jang A-S (2017) Effect of TiO2 nanoparticles on inflammasome-mediated airway inflammation and responsiveness. Allergy Asthma Immunol Res 9:257–264
Ko J-W et al (2016) Copper oxide nanoparticle induces inflammatory response and mucus production via MAPK signaling in human bronchial epithelial cells. Environ Toxicol Pharmacol 43:21–26
Kojima S et al (2018) Recovery from rheumatoid arthritis following 15 months of therapy with low doses of ionizing radiation: a case report. Dose Response 16:1559325818784719
Li J et al (2019) Safety and efficacy of pulsed low-dose rate radiotherapy for local recurrent esophageal squamous cell carcinoma after radiotherapy: study protocol for a prospective multi-center phase II trial. Medicine (baltimore) 98:e16176
Ma-Hock L et al (2009) Development of a short-term inhalation test in the rat using nano-titanium dioxide as a model substance. Inhal Toxicol 21:102–118
Mirzaie-Joniani H et al (2002) Apoptosis induced by low-dose and low-dose-rate radiation. Cancer: Interdiscip Int J Am Cancer Soc. 94:1210–1214
Moghimi SM, Hunter AC, Murray JC (2005) Nanomedicine: current status and future prospects. FASEB J 19:311–330
Moradian N et al (2020) Cytokine release syndrome: inhibition of pro-inflammatory cytokines as a solution for reducing COVID-19 mortality. Eur Cytokine Netw 31:81–93
Park H-R, Jo S-K, Yu D-K, Jung U (2013) Fractionated irradiations lead to chronic allergic airway inflammation through increasing the influx of macrophages. Inflamm Res 62:27–36
Park J-W et al (2016) Copper oxide nanoparticles aggravate airway inflammation and mucus production in asthmatic mice via MAPK signaling. Nanotoxicology 10:445–452
Parnsamut C, Brimson S (2015) Effects of silver nanoparticles and gold nanoparticles on IL-2, IL-6, and TNF-α production via MAPK pathway in leukemic cell lines. Genet Mol Res 14:3650–3668
Ren H et al (2006) Augmentation of innate immunity by low-dose irradiation. Cell Immunol 244:50–56
Renda T et al (2008) Increased activation of p38 MAPK in COPD. Eur Respir J 31:62–69
Saito A, Horie M, Nagase T (2018) TGF-β signaling in lung health and disease. Int J Mol Sci 19:2460
Saqib U et al (2018) Phytochemicals as modulators of M1–M2 macrophages in inflammation. Oncotarget 9:17937
Schleh C et al (2009) The effect of titanium dioxide nanoparticles on pulmonary surfactant function and ultrastructure. Respir Res 10:90
Schröder C et al (2020) Impact of low-dose irradiation of the lung and heart on toxicity and pulmonary function parameters after thoracic radiotherapy. Cancers (basel) 13:22
Shimura N, Kojima S (2014) Effects of low-dose-gamma rays on the immune system of different animal models of disease. Dose Response 12:429–465
Shin IS et al (2015) Melatonin attenuates neutrophil inflammation and mucus secretion in cigarette smoke-induced chronic obstructive pulmonary diseases via the suppression of Erk-Sp1 signaling. J Pineal Res 58:50–60
Shin N-R et al (2018) So-Cheong-Ryoung-Tang attenuates pulmonary inflammation induced by cigarette smoke in bronchial epithelial cells and experimental mice. Front Pharmacol 9:1064
Tang FR, Loke WK, Khoo BC (2017) Low-dose or low-dose-rate ionizing radiation-induced bioeffects in animal models. J Radiat Res 58:165–182
Underwood DC et al (2000) SB 239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am J Physiol Lung Cell Mol Physiol 279:L895-902
UNSCEAR (2000) The United Nations scientific committee on the effects of atomic radiation. Health Phys 79:314–314
Wang J, Chen W-D, Wang Y-D (2020) The relationship between gut microbiota and inflammatory diseases: the role of macrophages. Front Microbiol 11:1065
Wu Z, He D, Zhao S, Wang H (2019) IL-17A/IL-17RA promotes invasion and activates MMP-2 and MMP-9 expression via p38 MAPK signaling pathway in non-small cell lung cancer. Mol Cell Biochem 455:195–206
Xu X et al (2019) Novel benzofuran derivative DK-1014 attenuates lung inflammation via blocking of MAPK/AP-1 and AKT/mTOR signaling in vitro and in vivo. Sci Rep 9:1–13
Yablecovitch D et al (2019) Serum MMP-9: a novel biomarker for prediction of clinical relapse in patients with quiescent Crohn’s disease, a post hoc analysis. Therap Adv Gastroenterol 12:1756284819881590
Acknowledgements
We thank Dr. MY Kim and Mr. JK Park for their administrative support.
Funding
This work was supported by a Dongnam Institute of Radiological & Medical Sciences grant funded by the Korean government Ministry of Science and ICT (MSIT) [grant numbers 50492–2016 and 50491–2022] and grants from the National Research Foundation funded by the MSIT [grant numbers NRF-2020M2C8A2069337, NRF-2020R1A2C1004272, and NRF-2020M2C8A2069351].
Author information
Authors and Affiliations
Contributions
SK: Formal analysis (lead); Investigation (equal); Writing–original draft (equal); Writing–review and editing (equal). H-JL: Data curation (equal); Formal analysis (equal); Investigation (lead). YS: Formal analysis (equal); Investigation (equal). WSJ: Data curation (equal); Formal analysis (equal). MJB: Methodology (Radiation exposure). JHP: Data curation (equal). SJ: Investigation (Histopathological exam). CM: Data curation (equal); Formal analysis (equal); Investigation (equal); Writing–original draft (lead); Writing–review and editing (equal). CGL and J-SK: Conceptualization (lead); Data curation (equal); Methodology (equal); Writing–original draft (lead); Writing–review and editing (equal).
Corresponding authors
Ethics declarations
Conflict of interest
Sohi Kang declares that she has no conflict of interest. Hae-June Lee declares that she has no conflict of interest. Yeonghoon Son declares that he has no conflict of interest. Min Ji Bae declares that she has no conflict of interest. Wol Soon Jo declares that she has no conflict of interest. Jun Hong Park declares that he has no conflict of interest. Sohee Jeong declares that she has no conflict of interest. Changjong Moon declares that he has no conflict of interest. In-Sik Shin declares that he has no conflict of interest. Chang Geun Lee declares that he has no conflict of interest. Joong Sun Kim declares that he has no conflict of interest.
Ethical approval
All animal experiments followed a protocol approved by the Institutional Animal Care and Use Committee of the Dongnam Institute of Radiological & Medical Science (DI-2016–002 and DI-2022–014).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, S., Lee, HJ., Son, Y. et al. Low-dose-rate gamma radiation aggravates titanium dioxide nanoparticle-induced lung injury in mice. Mol. Cell. Toxicol. 20, 389–398 (2024). https://doi.org/10.1007/s13273-023-00353-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-023-00353-2