Abstract
Objectives
This study aimed at investigating the role of the proprotein convertase subtilisin/Kexin type 9 (PCSK9)-mediated autophagy on myocardial ischemia/reperfusion injury (MIRI). To determine the relationship between autophagy, apoptosis, fibrosis, and inflammation in the myocardium, to provide experience in preventing and treating the myocardial ischemia/reperfusion (I/R) injury.
Methods
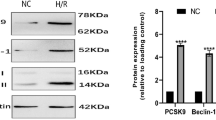
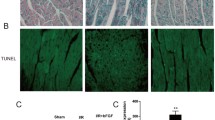
An AC16 hypoxia-reoxygenation model and a rat myocardial ischemia–reperfusion model were established. The concentrations of cardiac troponin T (cTnT) and creatine kinase-MB (CKMB) in plasma were measured by ELISA. To determine the size of the myocardial infarction, TTC/EB staining was performed. In addition to identifying pathological changes in myocardial tissue, Masson’s trichrome stains and H&E stains were used to identify pathological changes. Echocardiography was employed to detect cardiac function. Western blot analysis was then performed to detect the protein expression of Parkin, Pink1, and markers associated with autophagy (Beclin-1, p62, LC3).
Results
A significant increase in PCSK9 was observed in the myocardium during H/R. In the cardiac-specific PCSK9 knockdown model, cardiac autophagy was significantly inhibited, whereas cardiac-specific PCSK9 overexpression promoted cardiac autophagy. In vivo studies have demonstrated a significant decrease in cardiac autophagy when the PCSK9 inhibitor was administered. Apoptosis induced by I/R was greatly decreased, and myocardial infarction size and function were both improved by PCSK9 inhibitors. Mechanistically, the PCSK9 inhibitor improved the degree of myocardial fibrosis and inhibited the development of inflammation.
Conclusions
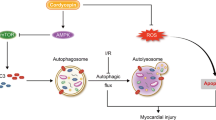
Our results demonstrated that increased PCSK9 via the parkin/pink1 signaling pathway contributes to I/R and H/R by exaggerating excessive autophagy during reperfusion/reoxygenation. In addition, the PCSK9 inhibitor blocked the development of inflammation and improved Infarct size, myocardial function, and myocardial fibrosis.






Similar content being viewed by others
Availability of supporting data
All data, models, or code generated or used during the study are available from the corresponding author by request.
Code availability
Not applicable.
References
Annemarie W (2020) Autocrine effects of PCSK9 on cardiomyocytes. Basic Res Cardiol 115(6):65
Ashraf MI et al (2014) A p38MAPK/MK2 signaling pathway leading to redox stress, cell death and ischemia/reperfusion injury. Cell Commun Signal 12:6
Cadenas S (2018) ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med 117:76–89
Chaofu Ke (2019) Letter by Ke and Shen Regarding Article, “Long-Term Association of Low-Density Lipoprotein Cholesterol With Cardiovascular Mortality in Individuals at Low 10-Year Risk of Atherosclerotic Cardiovascular Disease: Results From the Cooper Center Longitudinal Study.” Circulation 139(18):2190–2191
Chen HY et al (2021) ELAVL1 is transcriptionally activated by FOXC1 and promotes ferroptosis in myocardial ischemia/reperfusion injury by regulating autophagy. Mol Med 27(1):14
Ding Z et al (2018) PCSK9 expression in the ischaemic heart and its relationship to infarct size, cardiac function, and development of autophagy. Cardiovasc Res 114(13):1738–1751
Han J-Y et al (2019) Effects and mechanisms of QiShenYiQi pills and major ingredients on myocardial microcirculatory disturbance, cardiac injury and fibrosis induced by ischemia-reperfusion. Pharmacol Res 147:104386
Hao Z (2018) Ripk3 regulates cardiac microvascular reperfusion injury: The role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration. Cell Signal 45:12–22
Huang G et al (2022) PCSK9 inhibition protects against myocardial ischemia-reperfusion injury via suppressing autophagy. Microvasc Res 142:104371
Li L et al (2020) Protective mechanism and clinical application of hydrogen in myocardial ischemia-reperfusion injury. Pak J Biol Sci 23(2):103–112
Narendra D et al (2008) Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 183(5):795–803
Ning Ke et al (2020) ATP-sensitive potassium channels mediate the cardioprotective effect of panax notoginseng saponins against myocardial ischaemia-reperfusion injury and inflammatory reaction. Biomed Res Int 2020:3039184
Passino C et al (2015) Markers of fibrosis, inflammation, and remodeling pathways in heart failure. Clinica Chimica Acta. Int J Clin Chem 443:29–38
Schreckenberg R et al (2022) Proprotein convertase subtilisin kexin type 9 (PCSK9) deletion but not inhibition of extracellular PCSK9 reduces infarct sizes ex vivo but not in vivo. Int J Mol Sci 23(12):6512
Shi B et al (2019) mTOR and Beclin1: Two key autophagy-related molecules and their roles in myocardial ischemia/reperfusion injury. J Cell Physiol 234(8):12562–12568
Siripong P (2019) PCSK9 inhibitor improves cardiac function and reduces infarct size in rats with ischaemia/reperfusion injury: benefits beyond lipid-lowering effects. J Cell Mol Med 23(11):7310–7319
Sun X et al (2021) Alda-1 treatment promotes the therapeutic effect of mitochondrial transplantation for myocardial ischemia-reperfusion injury. Bioact Mater 6(7):2058–2069
Wang J, Toan S, Zhou H (2020) New insights into the role of mitochondria in cardiac microvascular ischemia/reperfusion injury. Angiogenesis 23(3):299–314
Xiang M et al (2021) Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Oxid Med Cell Longev 2021:6614009
Xie W, Zhou J (2018) Aberrant regulation of autophagy in mammalian diseases. Biol Lett 14(1):20170540
Yang M et al (2019) Mitophagy and mitochondrial integrity in cardiac ischemia-reperfusion injury. Biochim Biophys Acta Mol Basis Dis 1865(9):2293–2302
Yellon DM, Hausenloy DJ (2007) Myocardial reperfusion injury. N Engl J Med 357:1121–1135
Yuan H et al (2009) LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am J Physiol Heart Circ Physiol 296(2):H470–H479
Zhang C et al (2006) TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol 26(3):475–480
Zhang W et al (2017) Mitophagy receptor FUNDC1 regulates mitochondrial homeostasis and protects the heart from I/R injury. Autophagy 13(6):1080–1081
Acknowledgement
This work was supported by a Research Grant of Shanghai University of Traditional Chinese Medicine and by National Natural Science Foundation grant funded by the China government (Grant/Award Number: 81303256). Following are results of study on the “ATP-Sensitive Potassium Channels Mediate the Cardioprotective Effect of Panax notoginseng Saponins against Myocardial Ischaemia–Reperfusion Injury and Inflammatory Reaction”.
Funding
This work was mainly supported by the Science and Technology Foundation of Health commission of Guizhou Province (Grant No. gzwkj2022-317、gzwkj2021-112); the National Natural Science Foundation of China (Nos. 82160086, 81960047); the Science and Technology Fund of Guizhou Provincial Health Department [qiankehepingtairencai-GCC [2022]040-, qiankehezhicheng [2019]2800]; the Health and Family Planning Commission of Guizhou Province (qianweijianhan [2021]160).
Author information
Authors and Affiliations
Contributions
GWH, HLB, and WL designed and performed experiments, analyzed and interpreted data, and prepared the manuscript. PZ, ZGD, XLX, MZL, BW, HXA, and LDXL participated in the design of the study and performed the statistical analysis. HYZ and ZHL conceived of the study and participated in its design and coordination and review of this manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Author Guangwei Huang declares that he/she has no conflict of interest; author Hailong Bao declares that he/she has no conflict of interest; author Peng Zhan declares that he/she has no conflict of interest; author Xiyang Lu declares that he/she has no conflict of interest; author Zonggang Duan declares that he/she has no conflict of interest; author Xinlin Xiong declares that he/she has no conflict of interest; author Muzhi Lin declares that he/she has no conflict of interest; author Bing Wang declares that he/she has no conflict of interest; author Hongxin An declares that he/she has no conflict of interest; author Luanda Xiahou declares that he/she has no conflict of interest; author Haiyan Zhou declares that he/she has no conflict of interest; author Zhenhua Luo declares that he/she has no conflict of interest, author Wei Li declares that he/she has no conflict of interest.
Ethics approval and consent to participate
Authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were approved by the Animal Experimentation Committee of the Institutional Review Board of Guizhou Medical University (License No. 2019 (105)) and complied with the guidelines of Guizhou Medical University for the care and use of animals. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the anshun city science and technology plan project.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, G., Bao, H., Zhan, P. et al. PCSK9 regulates myocardial ischemia–reperfusion injury through parkin/pink1-mediated autophagy pathway. Mol. Cell. Toxicol. 20, 367–376 (2024). https://doi.org/10.1007/s13273-023-00352-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-023-00352-3