Abstract
Background
Polyphyllin I (PPI), a steroidal saponin, exhibits antitumor activity and chemosensitization effect for a broad spectrum of cancer cells, however, its toxicity and chemosensitization effect in vivo is still unknown.
Objective
We investigated PPI’s cytotoxic activity, toxicity and chemosensitization effect and in vitro and in vivo.
Results
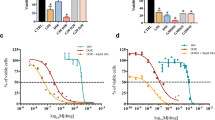
The IC50 values of PPI on MCF-7, H22, and S180 tumor cells were 4.37 µmol/L, 1.71 µmol/L, and 0.92 µmol/L, respectively. The LD50 of PPI was found to be 47.9 mg/kg using ip. injection. PPI at concentrations of 0.3 mg/kg, 0.6 mg/kg, 1.2 mg/kg, and 2.4 mg/kg (1/80 LD50–1/20 LD50) were synergized with DOX of 0.5 mg/kg to inhibit the H22 and S180 tumor growth in vivo by inducing apoptosis without obvious immunotoxicity. PPI exhibited a remarkable hemolytic effect on rabbit erythrocytes (EC50 = 4.3 µM), while it had no impact in mice.
Conclusion
Our study revealed that the PPI-sensitized chemotherapeutic effect, when used in safe doses, circumvents immunotoxic side effects of DOX in vivo; thus, helping future clinical research.







Similar content being viewed by others
Abbreviations
- PPI:
-
Polyphyllin I
- DOX:
-
Doxorubicin
- NS:
-
Normal saline
- MTT:
-
3-(4, 5-Dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide
- ip.:
-
Intraperitoneal
- HE:
-
Hematoxylin–eosin
- TUNEL:
-
TdT-mediated dUTP nick-end labeling
- ALP:
-
Alkaline phosphatase
- RBC:
-
Red blood cell
- TBILT:
-
Total bilirubin
- DBILT:
-
Direct bilirubin
- IBILT:
-
Indirect bilirubin
References
Al Sawah E, Marchion DC, Xiong Y, Ramirez IJ, Abbasi F, Boac BM, Bush SH, Bou Zgheib N, McClung EC, Khulpateea BR, Berry A, Hakam A, Wenham RM, Lancaster JM, Judson PL (2015) The Chinese herb polyphyllin D sensitizes ovarian cancer cells to cisplatin-induced growth arrest. J Cancer Res Clin Oncol 141:237–242. https://doi.org/10.1007/s00432-014-1797-x
Al-Malky HS, Al Harthi SE, Osman AM (2020) Major obstacles to doxorubicin therapy: cardiotoxicity and drug resistance. J Oncol Pharm Pract 26:434–444. https://doi.org/10.1177/1078155219877931
Awad MG, Ali RA, Abd El-Monem DD, El-Magd MA (2020) Graviola leaves extract enhances the anticancer effect of cisplatin on various cancer cell lines. Mol Cell Toxicol 16:385–399. https://doi.org/10.1007/s13273-020-00092-8
Chen Z, Duan H, Tong X, Hsu P, Han L, Morris-Natschke SL, Yang S, Liu W, Lee KH (2018) Cytotoxicity, hemolytic toxicity, and mechanism of action of pulsatilla saponin D and its synthetic derivatives. J Nat Prod 81:465–474. https://doi.org/10.1021/acs.jnatprod.7b00578
Deng X, Luo S, Luo X, Hu M, Ma F, Wang Y, Zhou L, Huang R (2018) Fraction from lycium barbarum polysaccharides reduces immunotoxicity and enhances antitumor activity of doxorubicin in mice. Integr Cancer Ther 17:860–866. https://doi.org/10.1177/1534735417753544
Dong R, Guo J, Zhang Z, Zhou Y, Hua Y (2018) Polyphyllin I inhibits gastric cancer cell proliferation by downregulating the expression of fibroblast activation protein alpha (FAP) and hepatocyte growth factor (HGF) in cancer-associated fibroblasts. Biochem Biophys Res Commun 497:1129–1134. https://doi.org/10.1016/j.bbrc.2018.02.193
Gao M, Cheung KL, Lau IP, Yu WS, Fung KP, Yu B, Loo JF, Kong SK (2012) Polyphyllin D induces apoptosis in human erythrocytes through Ca2+ rise and membrane permeabilization. Arch Toxicol 86:741–752. https://doi.org/10.1007/s00204-012-0808-4
Jin ZH, Furukawa T, Ohya T, Degardin M, Sugyo A, Tsuji AB, Fujibayashi Y, Zhang MR, Higashi T, Boturyn D, Dumy P, Saga T (2017) 67Cu-Radiolabeling of a multimeric RGD. peptide for αVβ3 integrin-targeted radionuclide therapy: stability, therapeutic efficacy, and safety studies in mice. Nucl Med Commun 38:347–355. https://doi.org/10.1097/mnm.0000000000000646
Lee MS, Yuet-Wa JC, Kong SK, Yu B, Eng-Choon VO, Nai-Ching HW, Chung-Wai TM, Fung KP (2005) Effects of polyphyllin D, a steroidal saponin in Paris polyphylla, in growth inhibition of human breast cancer cells and in xenograft. Cancer Biol Ther 4:1248–1254. https://doi.org/10.4161/cbt.4.11.2136
Li L, Wu J, Zheng F, Tang Q, Wu W, Hann SS (2016) Inhibition of EZH2 via activation of SAPK/JNK and reduction of p65 and DNMT1 as a novel mechanism in inhibition of human lung cancer cells by polyphyllin I. J Exp Clin Cancer Res 35:112. https://doi.org/10.1186/s13046-016-0388-x
Li K, Liu W, Zhao Q, Wu C, Fan C, Lai H, Li S (2019) Combination of tanshinone IIA and doxorubicin possesses synergism and attenuation effects on doxorubicin in the treatment of breast cancer. Phytother Res 33:1658–1669. https://doi.org/10.1002/ptr.6353
Li GB, Fu RQ, Shen HM, Zhou J, Hu XY, Liu YX, Li YN, Zhang HW, Liu X, Zhang YH, Huang C, Zhang R, Gao N (2017) Polyphyllin I induces mitophagic and apoptotic cell death in human breast cancer cells by increasing mitochondrial PINK1 levels. Oncotarget 8:10359–10374. https://doi.org/10.18632/oncotarget.14413
Liu J, Man S, Liu Z, Ma L, Gao W (2016) A synergistic antitumor effect of polyphyllin I and formosanin C on hepatocarcinoma cells. Bioorg Med Chem Lett 26:4970–4975. https://doi.org/10.1016/j.bmcl.2016.09.005
Lou W, Chen Y, Zhu KY, Deng H, Wu T, Wang J (2017) Polyphyllin I overcomes EMT-associated resistance to erlotinib in lung cancer cells via IL-6/STAT3 pathway inhibition. Biol Pharm Bull 40:1306–1313. https://doi.org/10.1248/bpb.b17-00271
Pajic M, Iyer JK, Kersbergen A, van der Burg E, Nygren AO, Jonkers J, Borst P, Rottenberg S (2009) Moderate increase in Mdr1a/1b expression causes in vivo resistance to doxorubicin in a mouse model for hereditary breast cancer. Can Res 69:6396–6404. https://doi.org/10.1158/0008-5472.Can-09-0041
Song S, Du L, Jiang H, Zhu X, Li J, Xu J (2016) Paris Saponin I sensitizes gastric cancer cell lines to cisplatin via cell cycle arrest and apoptosis. Med Sci Monit 22:3798–3803. https://doi.org/10.1265/msm.898232
Tian Y, Gong GY, Ma LL, Wang ZQ, Song D, Fang MY (2020) Anticancer effects of Polyphyllin I: an update in 5 years. Chem Biol Interact 316:108936. https://doi.org/10.1016/j.cbi.2019.108936
Wang YH, Shi M, Niu HM, Yang J, Xia MY, Luo JF, Chen YJ, Zhou YP, Li H (2018) Substituting one Paris for another? In vitro cytotoxic and in vivo antitumor activities of Paris forrestii, a substitute of Paris polyphylla var. yunnanensis. J Ethnopharmacol 218:45–50. https://doi.org/10.1016/j.jep.2018.02.022
Wang Y, Li X, Liu X, Chen Y, Yang C, Tan C, Wang B, Sun Y, Zhang X, Gao Y, Ding J, Meng L (2019) Simultaneous inhibition of PI3Kα and CDK4/6 synergistically suppresses KRAS-mutated non-small cell lung cancer. Cancer Biol Med 16:66–83. https://doi.org/10.20892/j.issn.2095-3941.2018.0361
Watanabe T, Naito M, Kokubu N, Tsuruo T (1997) Regression of established tumors expressing P-glycoprotein by combinations of adriamycin, cyclosporin derivatives, and MRK-16 antibodies. J Natl Cancer Inst 89:512–518. https://doi.org/10.1093/jnci/89.7.512
Wei T, Xiaojun X, Peilong C (2020) Magnoflorine improves sensitivity to doxorubicin (DOX) of breast cancer cells via inducing apoptosis and autophagy through AKT/mTOR and p38 signaling pathways. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2019.109139
Xiao T, Zhong W, Zhao J, Qian B, Liu H, Chen S, Qiao K, Lei Y, Zong S, Wang H, Liang Y, Zhang H, Meng J, Zhou H, Sun T, Liu Y, Yang C (2018) Polyphyllin I suppresses the formation of vasculogenic mimicry via Twist1/VE-cadherin pathway. Cell Death Dis 9:906. https://doi.org/10.1038/s41419-018-0902-5
Yang Q, Chen W, Xu Y, Lv X, Zhang M, Jiang H (2018) Polyphyllin I modulates MALAT1/STAT3 signaling to induce apoptosis in gefitinib-resistant non-small cell lung cancer. Toxicol Appl Pharmacol 356:1–7. https://doi.org/10.1016/j.taap.2018.07.031
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81960739 and 32070361), by grants from the Joint Application and Basic Research Foundation of Kunming Medical University & Science and Technology Department of Yunnan Province of China (grant no. 2019FE001(-193) and 202001AY070001–180), Yunnan Major Biopharmaceutical Project (grant no. 2018ZF002), Digitalization, development, and application of biotic resources of Kunming Medical University (202002AA100007), the seventh batch of Yunnan specialty plant polysaccharide engineering research center construction plan (2019–57) and Innovative Research Team Program in Science and Technology in Kunming Medical University (CXTD202003).
Author information
Authors and Affiliations
Contributions
Z-X, N-X, Z-YQ, X-YAT, C-DY, M-JQ, Z-Y and Y-FH involved in design, data collection, writing, and revising the manuscript. Z-YP, Z-X, N-X, Z-YQ and Y-HZ involved in conception, design, literature review, writing the manuscript. Z-YP and W-YH involved in design, writing, revising the manuscript. In addition, all the authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests. The author Xiang Zhu declares that he has no conflict of interest; the author Xin Na declares that he has no conflict of interest; the author Yueqin Zeng declares that she has no conflict of interest; the author Yangantai Xu declares that he has no conflict of interest; the author Dongya Chai declares that she has no conflict of interest; the author Huanzhi Yang declares that she has no conflict of interest; the author Jingqian Miao declares that she has no conflict of interest; the author Yuan Zhang declares that she has no conflict of interest; the author Fenghua Yang declares that she has no conflict of interest; the author Yuehu Wang declares that he has no conflict of interest; the author Yiping Zhou declares that she has no conflict of interest.
Ethical approval
All the animal experiments were conducted following the guidelines of China Council on Animal Care and Use. The study was approved by Ethical Committee of Kunming Medical University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, X., Na, X., Zeng, Y. et al. Polyphyllin I combined with doxorubicin shows chemosensitization effect in vivo and reduces immunotoxicity of doxorubicin. Mol. Cell. Toxicol. 18, 359–369 (2022). https://doi.org/10.1007/s13273-021-00206-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-021-00206-w