Abstract
Backgrounds
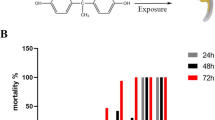
3,4-Dichloroaniline (3,4-DCA) is a transformation product of herbicides that is commonly used as a reference in developmental toxicity studies (OECD TG 236) (Bonnet et al. in Environ Toxicol 22:78–91, 2007). However, the mechanisms underlying 3,4-DCA-induced hepatotoxicity are not well known.
Methods
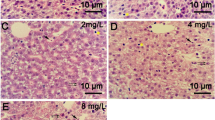
We exposed zebrafish larvae at 72 hpf to 3,4-DCA for 3 days and observed lipid accumulation in liver treated with 10-μM 3,4-DCA using oil red O staining. Subsequently, we performed qRT-PCR analysis to determine the genes involved in the observed lipid accumulation.
Results
We found that genes related to lipogenesis (srebp1, pparγ, lipin1, and scd1) and ER stress (bip, atf4, ddit3, dnajc3, and edem1) were significantly upregulated. In addition, we found that ROS generation increased in the larvae treated with 10-μM 3,4-DCA. Moreover, glutathione-S-transferase activity in these larvae was increased by 3,4-DCA in a dose-dependent manner, and the expression of the inflammation marker il-1β increased.
Conclusion
Our results indicated that exposure to 3,4-DCA induced fatty liver in zebrafish larvae and that this, in association with additional factors such as ER stress response, can promote liver damage. We accordingly suggest that 3,4-DCA could be used to induce fatty liver in zebrafish larvae.




Similar content being viewed by others
References
Amali AA et al (2006) Thioacetamide induced liver damage in zebrafish embryo as a disease model for steatohepatitis. J Biomed Sci 13:225–232
Antherieu S, Rogue A, Fromenty B, Guillouzo A, Robin MA (2011) Induction of vesicular steatosis by amiodarone and tetracycline is associated with up-regulation of lipogenic genes in HepaRG cells. Hepatology 53:1895–1905
Asaoka Y, Terai S, Sakaida I, Nishina H (2013) The expanding role of fish models in understanding non-alcoholic fatty liver disease. Dis Model Mech 6:905–914
Bonnet JL, Bonnemoy F, Dusser M, Bohatier J (2007) Assessment of the potential toxicity of herbicides and their degradation products to nontarget cells using two microorganisms, the bacteria Vibrio fischeri and the ciliate Tetrahymena pyriformis. Environ Toxicol 22:78–91
Browning JD, Horton JD (2004) Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 114:147–152
Cinaroglu A, Gao C, Imrie D, Sadler KC (2011) Activating transcription factor 6 plays protective and pathological roles in steatosis due to endoplasmic reticulum stress in zebrafish. Hepatology 54:495–508
Cobbina E, Akhlaghi F (2017) Non-alcoholic fatty liver disease (NAFLD)—pathogenesis, classification, and effect on drug metabolizing enzymes and transporters. Drug Metab Rev 49:197–211
Dai W et al (2015) High fat plus high cholesterol diet lead to hepatic steatosis in zebrafish larvae: a novel model for screening anti-hepatic steatosis drugs. Nutr Metab 12:42
Ferre P, Foufelle F (2007) SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res 68:72–82
Field HA, Ober EA, Roeser T, Stainier DY (2003) Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev Biol 253:279–290
Guengerich FP, Munro AW (2013) Unusual cytochrome p450 enzymes and reactions. J Biol Chem 288:17065–17073
Guillen N et al (2009) Microarray analysis of hepatic gene expression identifies new genes involved in steatotic liver. Physiol Genomics 37:187–198
Hardwick RN, Fisher CD, Canet MJ, Lake AD, Cherrington NJ (2010) Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 38:2293–2301
Higuchi N et al (2008) Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol Res 38:1122–1129
Hill A, Mesens N, Steemans M, Xu JJ, Aleo MD (2012) Comparisons between in vitro whole cell imaging and in vivo zebrafish-based approaches for identifying potential human hepatotoxicants earlier in pharmaceutical development. Drug Metab Rev 44:127–140
Ji C et al (2011) Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology 54:229–239
Kamari Y et al (2011) Lack of interleukin-1alpha or interleukin-1beta inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol 55:1086–1094
Lawler JM, Song W, Demaree SR (2003) Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med 35:9–16
Lebeaupin C et al (2018) Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol 69:927–947
Lee JS et al (2012) Pharmacologic ER stress induces non-alcoholic steatohepatitis in an animal model. Toxicol Lett 211:29–38
Li WM, Yin DQ, Zhou Y, Hu SQ, Wang LS (2003) 3,4-dichloroaniline-induced oxidative stress in liver of crucian carp (Carassius auratus). Ecotoxicol Environ Saf 56:251–255
Malhi H, Kaufman RJ (2011) Endoplasmic reticulum stress in liver disease. J Hepatol 54:795–809
Malhotra JD, Kaufman RJ (2007) Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9:2277–2293
McGrath P, Li CQ (2008) Zebrafish: a predictive model for assessing drug-induced toxicity. Drug Discov Today 13:394–401
Monteiro M et al (2006) Acute effects of 3,4-dichloroaniline on biomarkers and spleen histology of the common goby Pomatoschistus microps. Chemosphere 62:1333–1339
OECD Guidelines for the Testing of Chemicals, Section 2, Test No. 236: Fish Embryo Acute Toxicity (FET) Test, http://www.oecd-ilibrary.org/environment/test-no-236-fish-embryo-acute-toxicity-fet-test_9789264203709-en (2013)
Peterson TR et al (2011) mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146:408–420
Pettinelli P et al (2009) Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim Biophys Acta 1792:1080–1086
Pham DH, Zhang C, Yin C (2017) Using zebrafish to model liver diseases—where do we stand? Curr Pathobiol Rep 5:207–221
Rutkowski DT et al (2008) UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell 15:829–840
Tolosa L et al (2016) Advantageous use of HepaRG cells for the screening and mechanistic study of drug-induced steatosis. Toxicol Appl Pharmacol 302:1–9
Vacaru AM et al (2014) Molecularly defined unfolded protein response subclasses have distinct correlations with fatty liver disease in zebrafish. Dis Model Mech 7:823–835
van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Wang C et al (2014) ATF4 deficiency protects hepatocytes from oxidative stress via inhibiting CYP2E1 expression. J Cell Mol Med 18:80–90
Wang S, Miller SR, Ober EA, Sadler KC (2017) Making it new again: insight into liver development, regeneration, and disease from Zebrafish research. Curr Top Dev Biol 124:161–195
Xu H et al (2016) Genome-wide identification of suitable zebrafish Danio rerio reference genes for normalization of gene expression data by RT-qPCR. J Fish Biol 88:2095–2110
Yeh KY et al (2017) ATF4 overexpression induces early onset of hyperlipidaemia and hepatic steatosis and enhances adipogenesis in zebrafish. Sci Rep 7:16362
Zhao F et al (2014) The effect and mechanism of tamoxifen-induced hepatocyte steatosis in vitro. Int J Mol Sci 15:4019–4030
Acknowledgements
Ji-Seon Park designed the project, performed experiments, prepared figures and wrote the manuscript. Jeongah Song, contributed to acquisition and analysis. Jong-Su Park, and Sangwoo Lee contributed to the zebrafish care, breeding, and embryo culture. Jieon Lee, Han-Jin Park and Woo-Keun Kim contributed to the guidance of experiments. Seokjoo Yoon reviewed and edited the manuscript. Hang-Suk Chun formulated ideas, designed the project, reviewed and edited the manuscript. This work was supported by the National Research Foundation of Korea [NRF-2017R1C1B2007686].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Ji-Seon Park, Jeongah Song, Jong-Su Park, Sangwoo Lee, Jieon Lee, Han-Jin Park, Woo-Keun Kim, Seokjoo Yoon, Hang-Suk Chun declared that have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, JS., Song, J., Park, JS. et al. 3,4-Dichloroaniline promotes fatty liver in zebrafish larvae. Mol. Cell. Toxicol. 16, 159–165 (2020). https://doi.org/10.1007/s13273-019-00066-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-019-00066-5