Abstract
Purpose
Wall shear stress (WSS) is a critically important physical factor contributing to atherosclerosis. Mapping the spatial distribution of local, oscillatory WSS can identify important mechanisms underlying the progression of coronary artery disease.
Methods
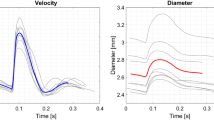
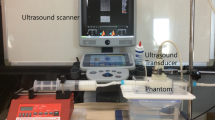
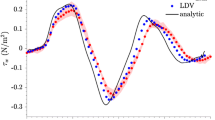
In this study, blood flow velocity and time-varying WSS were estimated in the left anterior descending (LAD) coronary artery of an ex vivo beating porcine heart using ultrasound with an 18 MHz linear array transducer aligned with the LAD in a forward-viewing orientation. A pulsatile heart loop with physiologically-accurate flow was created using a pulsatile pump. The coronary artery wall motion was compensated using a local block matching technique. Next, 2D and 3D velocity magnitude and WSS maps in the LAD coronary artery were estimated at different time points in the cardiac cycle using an ultrafast Doppler approach. The blood flow velocity estimated using the presented approach was compared with a commercially-available, calibrated single element blood flow velocity measurement system.
Results
The resulting root mean square error (RMSE) of 2D velocity magnitude acquired from a high frequency, linear array transducer was less than 8% of the maximum velocity estimated by the commercial system.
Conclusion
When implemented in a forward-viewing intravascular ultrasound device, the presented approach will enable dynamic estimation of WSS, an indicator of plaque vulnerability in coronary arteries.






Similar content being viewed by others
References
Tsao, C. W., et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 145:e153–e639, 2022.
Eshtehardi, P., et al. High wall shear stress and high-risk plaque: an emerging concept. Int. J. Cardiovasc. Imaging. 33:1089–1099, 2017.
Koskinas, K. C., et al. The role of low endothelial shear stress in the conversion of atherosclerotic lesions from stable to unstable plaque. Curr. Opin. Cardiol. 24:580–590, 2009.
Fukumoto, Y., et al. Localized elevation of shear stress is related to coronary plaque rupture: a 3-dimensional intravascular ultrasound study with in-vivo color mapping of shear stress distribution. J. Am. Coll. Cardiol. 51:645–650, 2008.
Kandangwa, P., et al. Influence of right coronary artery motion, flow pulsatility and non-Newtonian rheology on wall shear stress metrics. Front. in Bioeng. Biotechnol. 10:962687, 2022.
Ku, D. N., D. P. Giddens, C. K. Zarins, and S. Glagov. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 5:293–302, 1985.
Kolandavel, M. K., E.-T. Fruend, S. Ringgaard, and P. G. Walker. The effects of time varying curvature on species transport in coronary arteries. Ann. Biomed. Eng. 34:1820–1832, 2006.
Moore, J. E., Jr., et al. Fluid wall shear stress measurements in a model of the human abdominal aorta: oscillatory behavior and relationship to atherosclerosis. Atherosclerosis. 110:225–240, 1994.
Sakellarios, A., et al. Prediction of atherosclerotic disease progression using LDL transport modelling: a serial computed tomographic coronary angiographic study. Eur. Heart J. 18:11–18, 2017.
Toutouzas, K., et al. Accurate and reproducible reconstruction of coronary arteries and endothelial shear stress calculation using 3D OCT: comparative study to 3D IVUS and 3D QCA. Atherosclerosis. 240:510–519, 2015.
Gogas, B. D., et al. Feasibility of optical coherence tomography–derived computational fluid dynamics in calcified vessels to assess treatment with orbital atherectomy. JACC. 9:e65–e66, 2016.
Van Der Giessen, A. G., et al. Plaque and shear stress distribution in human coronary bifurcations: a multi-slice computed tomography study. In: Summer Bioengineering Conference, 2007, pp. 477–478.
Zhong, L., et al. Application of patient-specific computational fluid dynamics in coronary and intra-cardiac flow simulations: challenges and opportunities. Front. Physiol. 9:742, 2018.
Masaryk, A. M., et al. In vitro and in vivo comparison of three MR measurement methods for calculating vascular shear stress in the internal carotid artery. Am. J. Neuroradiol. 20:237–245, 1999.
Fonken, J., et al. The impact of a limited field-of-view on computed hemodynamics in abdominal aortic aneurysms: evaluating the feasibility of completing ultrasound segmentations with parametric geometries. Ann. Biomed. Eng. 51:1–14, 2023.
Deng, Z., et al. Noninvasive measurement of pressure gradient across a coronary stenosis using phase contrast (PC)-MRI: a feasibility study. Magn. Reson. Med. 77:529–537, 2017.
Poelma, C., P. Vennemann, R. Lindken, and J. Westerweel. In vivo blood flow and wall shear stress measurements in the vitelline network. Exp. Fluids. 45:703–713, 2008.
Li, M., et al. High pulsatility flow induces adhesion molecule and cytokine mRNA expression in distal pulmonary artery endothelial cells. Ann. Biomed. Eng. 37:1082–1092, 2009.
Yiu, B. Y., and C. Alfred. Least-squares multi-angle Doppler estimators for plane-wave vector flow imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 63:1733–1744, 2016.
Haniel, J., et al. Efficacy of ultrasound vector flow imaging in tracking omnidirectional pulsatile flow. Med. Phys. 50:1699, 2022.
Ekroll, I. K., et al. An extended least squares method for aliasing-resistant vector velocity estimation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 63:1745–1757, 2016.
Chee, A. J., C. K. Ho, B. Y. Yiu, and C. Alfred. Time-resolved wall shear rate mapping using high-frame-rate ultrasound imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 69:3367–3381, 2022.
Udesen, J., and J. A. Jensen. Investigation of transverse oscillation method. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 53:959–971, 2006.
Lenge, M., et al. Plane-wave transverse oscillation for high-frame-rate 2-D vector flow imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 62:2126–2137, 2015.
Salles, S., et al. 2-D arterial wall motion imaging using ultrafast ultrasound and transverse oscillations. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 62:1047–1058, 2015.
Jensen, J., et al. Fast plane wave 2-D vector flow imaging using transverse oscillation and directional beamforming. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 64:1050–1062, 2017.
Aizawa, K., et al. Brachial artery vasodilatory response and wall shear rate determined by multigate Doppler in a healthy young cohort. J. Appl. Physiol. 124:150–159, 2018.
Ramalli, A., et al. Continuous simultaneous recording of brachial artery distension and wall shear rate: a new boost for flow-mediated vasodilation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 66:463–471, 2018.
Wang, I. C., H. Huang, W. T. Chang, and C. C. Huang. Wall shear stress mapping for human femoral artery based on ultrafast ultrasound vector Doppler estimations. Med. Phys. 48:6755–6764, 2021.
Poelma, C., et al. Ultrasound imaging velocimetry: toward reliable wall shear stress measurements. Eur. J. Mech. B. 35:70–75, 2012.
Correia, M., et al. Quantitative imaging of coronary flows using 3D ultrafast Doppler coronary angiography. Phys. Med. Biol. 65:105013, 2020.
Pinter, S. Z., et al. Evaluation of umbilical vein blood volume flow in preeclampsia by angle-independent 3D sonography. J. Ultrasound Med. 37:1633–1640, 2018.
Welsh, A. W., et al. Three-dimensional US fractional moving blood volume: validation of renal perfusion quantification. Radiology. 293:460–468, 2019.
Pinter, S. Z., et al. Volumetric blood flow in transjugular intrahepatic portosystemic shunt revision using 3-dimensional Doppler sonography. J. Ultrasound Med. 34:257–266, 2015.
Correia, M., J. Provost, M. Tanter, and M. Pernot. 4D ultrafast ultrasound flow imaging: in vivo quantification of arterial volumetric flow rate in a single heartbeat. Phys. Med. Biol. 61:L48, 2016.
Hong, J., et al. A dual-mode imaging catheter for intravascular ultrasound application. IEEE Trans. Med. Imaging. 38:657–663, 2018.
Janjic, J., et al. Sparse ultrasound image reconstruction from a shape-sensing single-element forward-looking catheter. IEEE Trans. Biomed. Eng. 65:2210–2218, 2018.
Kumar, V., et al. Unambiguous identification and visualization of an acoustically active catheter by ultrasound imaging in real time: theory, algorithm, and phantom experiments. IEEE Trans. Biomed. Eng. 65:1468–1475, 2017.
Kim, S., B. Jing, and B. D. Lindsey. Forward-viewing estimation of 3D blood flow velocity fields by intravascular ultrasound: influence of the catheter on velocity estimation in stenoses. Ultrasonics. 117:106558, 2021.
Kim, S., et al. Forward-viewing ultrasound-based wall shear stress estimation in coronary arteries: comparison with computational fluid dynamics (Submitted).
Lindsey, B. D., et al. 3-D intravascular characterization of blood flow velocity fields with a forward-viewing 2-D array. Ultrasound in Medicine Biology. 46:2560–2571, 2020.
Kumar, A., et al. High coronary shear stress in patients with coronary artery disease predicts myocardial infarction. J. Am. Coll. Cardiol. 72:1926–1935, 2018.
Shi, Y., F. J. de Ana, S. J. Chetcuti, and M. O’Donnell. Motion artifact reduction for IVUS-based thermal strain imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 52:1312–1319, 2005.
Leung, K. E., et al. Motion compensation for intravascular ultrasound palpography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 53:1269–1280, 2006.
Shiina, T., N. Nitta, H. Endo, and M. Yamagishi. Assessment of vulnerable coronary plaque by intravascular elasticity imaging. IEEE Ultrason. Symp. 2004:364–367, 2004.
Cormier, P., J. Porée, C. Bourquin, and J. Provost. Dynamic myocardial ultrasound localization angiography. IEEE Trans. Med. Imaging. 40:3379–3388, 2021.
Dilba, K., et al. The association between time-varying wall shear stress and the development of plaque ulcerations in carotid arteries from the plaque at risk study. Front. Cardiovasc. Med. 8:732646, 2021.
Agra, E. J., et al. Left ventricular thinning and distension in pig hearts as a reproducible ex vivo model of functional mitral regurgitation. ASAIO J. (1992). 66:1016, 2020.
Amedi, A., et al. Hemodynamic outcomes after undersizing ring annuloplasty and focal suture annuloplasty for surgical repair of functional tricuspid regurgitation. J. Thorac. Cardiovasc. Surg. 164:76-87 e71, 2022.
Bernard, A., et al. 3D echocardiographic reference ranges for normal left ventricular volumes and strain: results from the EACVI NORRE study. Eur. Heart J. 18:475–483, 2017.
Jing, B., M. E. Brown, M. E. Davis, and B. D. Lindsey. Imaging the activation of low-boiling-point phase-change contrast agents in the presence of tissue motion using ultrafast inter-frame activation ultrasound imaging. Ultrasound Med. Biol. 46:1474–1489, 2020.
Lindsey, B. D., et al. High resolution ultrasound superharmonic perfusion imaging: In vivo feasibility and quantification of dynamic contrast-enhanced acoustic angiography. Ann. Biomed. Eng. 45:939–948, 2017.
Newsome, I. G., T. M. Kierski, and P. A. Dayton. Assessment of the superharmonic response of microbubble contrast agents for acoustic angiography as a function of microbubble parameters. Ultrasound Med. Biol. 45:2515–2524, 2019.
Dewan, M., G. D. Hager, and C. H. Lorenz. Image-based coronary tracking and beat-to-beat motion compensation: feasibility for improving coronary MR angiography. Magn. Reson. Med. 60:604–615, 2008.
Zahnd, G., et al. Evaluation of a Kalman-based block matching method to assess the bi-dimensional motion of the carotid artery wall in B-mode ultrasound sequences. Med. Image Anal. 17:573–585, 2013.
De Korte, C., et al. Morphological and mechanical information of coronary arteries obtained with intravascular elastography. Feasibility study in vivo. Eur. Heart J. 23:405–413, 2002.
Pisters, R., et al. Instantaneous wave-free ratio and fractional flow reserve in clinical practice. Neth. Hear. J. 26:385–392, 2018.
Yeung, F., S. F. Levinson, and K. J. Parker. Multilevel and motion model-based ultrasonic speckle tracking algorithms. Ultrasound Med. Biol. 24:427–441, 1998.
Friemel, B. H., L. N. Bohs, and G. E. Trahey. Relative performance of two-dimensional speckle-tracking techniques: normalized correlation, non-normalized correlation and sum-absolute-difference. In: 1995 IEEE Ultrasonics Symposium. Proceedings. An International Symposium, 1995, pp. 1481–1484.
Bohs, L. N., and G. E. Trahey. A novel method for angle independent ultrasonic imaging of blood flow and tissue motion. IEEE Trans. Biomed. Eng. 38:280–286, 1991.
Danilouchkine, M. G., F. Mastik, and A. F. van der Steen. Accuracy in prediction of catheter rotation in IVUS with feature-based optical flow—a phantom study. IEEE Trans. Inf Technol. Biomed. 12:356–365, 2008.
Demené, C., et al. Spatiotemporal clutter filtering of ultrafast ultrasound data highly increases Doppler and fUltrasound sensitivity. IEEE Trans. Med. Imaging. 34:2271–2285, 2015.
Pihl, M. J., et al. A transverse oscillation approach for estimation of three-dimensional velocity vectors, part II: experimental validation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 61:1608–1618, 2014.
Jensen, J., M. B. Stuart, and J. A. Jensen. High frame rate vector velocity estimation using plane waves and transverse oscillation. In: 2015 Ieee International Ultrasonics Symposium (Ius), 2015, pp. 1–4.
Jensen, J. A. Comparison of vector velocity imaging using directional beamforming and transverse oscillation for a convex array transducer. In: Medical Imaging 2014: Ultrasonic Imaging and Tomography, 2014, pp. 279–286.
Kim, S., J. M. Tempestti, A. Veneziani, and B. D. Lindsey. A new method for estimating wall shear stress estimation using Doppler ultrasound imaging in coronary arteries. (In preparation).
Riemer, K., et al. Determining haemodynamic wall shear stress in the rabbit aorta in vivo using contrast-enhanced ultrasound image velocimetry. Ann. Biomed. Eng. 48:1728–1739, 2020.
Ge, J., et al. Intravascular ultrasound imaging of angiographically normal coronary arteries: a prospective study in vivo. Heart. 71:572–578, 1994.
Zeng, D., et al. A study on the compliance of a right coronary artery and its impact on wall shear stress. J. Biomech. Eng. 130:041014, 2008.
Jhunjhunwala, P., et al. Non-Newtonian blood flow in left coronary arteries with varying stenosis: a comparative study. Mol. Cell. Biomech. 13:1–21, 2016.
Doucette, J. W., et al. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 85:1899–1911, 1992.
Ofili, E. O., A. J. Labovitz, and M. J. Kern. Coronary flow velocity dynamics in normal and diseased arteries. Am. J. Cardiol. 71:D3–D9, 1993.
Acknowledgements
The authors thank John Oshinski for helpful discussions.
Funding
This work is supported by Grant R01EB031101 from the U.S. National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors report no conflicts of interest or competing interests.
Additional information
Associate Editor Diego Gallo oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 8829 kb)
Supplementary file2 (MP4 8184 kb)
Supplementary file3 (MP4 7708 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, S., Jing, B., Lane, B.A. et al. Dynamic Coronary Blood Flow Velocity and Wall Shear Stress Estimation Using Ultrasound in an Ex Vivo Porcine Heart. Cardiovasc Eng Tech 15, 65–76 (2024). https://doi.org/10.1007/s13239-023-00697-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-023-00697-9