Abstract
Purpose
The study was designed to evaluate the value of left atrial (LA) sphericity (LASP) in the identification of patients with atrial fibrillation (AF) who had prior ischemic stroke. The secondary aim was to investigate the possibility of improving stroke risk assessment based on six geometrical variables of LA.
Methods
This prospective observational study involved 157 patients: 74 in the stroke group and 83 in the control. All patients had cardiac computed tomography (CT) performed to analyze LA volume and dimensions. LASP and the discriminant function of six geometrical measurements were calculated.
Results
Multivariate logistic regression analysis showed a significant association of stroke with and gender, diabetes, CHA2DS2-VASc score, LA anteroposterior diameter, and LA sphericity. Patients with prior stroke had lower LASP than those without (66.6 ± 10.3% vs. 70.5 ± 7%; p = 0.0062). The most accurate identification of patients with a history of ischemic stroke was achieved by using a function of six geometrical measurements, the sphericity and volume coefficient. The C-statistic was higher for the above discriminant function (0.7273) than for LASP (0.3974). The addition of the discriminant function to the CHA2DS2-VASc score increased the performance of the risk score alone.
Conclusion
LASP is associated with prior stroke in AF patients. The proposed new formula for identification of AF patients who are at risk of stroke, based on geometrical measurements of LA, is superior to the basic LASP in identification of AF patients with a history of stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia, and has become an increasingly serious public health problem in an aging population. It doubles the risk of death and increases the risk of ischemic stroke fivefold.7, 14 Stroke is the main cause of long-term disability, and is a serious problem for healthcare systems.9 It has been estimated that 20% of ischemic strokes are caused by cardiac embolisms, most often in the course of AF.6
It is common practice to assess the risk of thromboembolic (TE) events in patients with AF by calculating the CHA2DS2-VASc score. However, multiple studies have shown that the score allows only moderately for the identification of patients at risk of stroke or peripheral embolism.5, 13 Thus, new factors are still being searched for to improve stratification of the risk of TE in the AF population.
The left atrial sphericity (LASP) is a novel parameter based on the analysis of the shape of the left atrium (LA) which depicts the difference between real LA geometry and a perfect sphere. The LA structural remodeling results in chamber dilation, alterations in the cardiomyocyte, fibroblast, and noncollagen infiltrative components of the atrium, but also changes in LA shape.3, 11 The LASP has been shown to be an independent predictor of arrhythmia recurrence after AF ablation. It may be also important in the identification of patients with an increased risk of TE, however, the data on this subject are limited.3, 4, 11
The primary aim of our study was to evaluate the value of LASP in identification of patients with AF who had prior ischemic stroke. The studied patient population was well-defined, with a known anticoagulation regimen at the time of neurological event. The secondary aim was to investigate the possibility of improving stroke risk assessment based on 6 geometrical variables derived from computed tomography (CT) of LA, using different statistical methods.
Methods
Patients and Study Design
The study design was previously described.2 Briefly, this prospective observational study included 74 consecutive randomly chosen patients after ischemic stroke, with AF, who were hospitalized in the neurological rehabilitation department after experiencing ischemic stroke between 2014 and 2016. The control group consisted of 83 randomly chosen subjects with AF, without a history of TE, scheduled for AF ablation in the cardiological ward (Fig. 1). In addition to the previously described exclusion criteria, patients with incomplete measurements of LA were also excluded from the present analysis. The study was approved by the local Ethical Committee of Centre of Postgraduate Medical Education and registered in the National Clinical Trial database (NCT02654795). Written informed consent was obtained from all patients.
Imaging Protocol
CT angiography was performed with a dual-source CT scanner (Somatom Definition Flash; Siemens Medical Solutions, Erlangen, Germany), using prospective ECG gating. High-concentration iodinated contrast material (≥ 350 mg/mL) was administered into the antecubital vein in the amount of 70–80 mL, using a power injector, at a flow rate of 5 mL/s, followed by a 30-mL saline chaser. Image acquisition was initiated 5 s after reaching 100 HU threshold enhancement within the region of interest placed in the left atrium. Prospective ECG-gated examinations were centered at the best diastolic phase of the R-R interval. Patients did not receive β-blockers to control heart rate.
Image Analysis
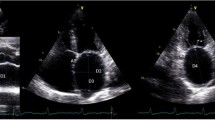
The analysis of anatomy and measurements of LA size were performed on 1-mm slices, using commercially available software (Leonardo, Siemens Medical Systems). The LA was compared to a fitted sphere according to Bisbal et al.4 The radius of the sphere which best fit the LA shape was calculated as the mean of distances between points of the LA wall in three planes, based on the principal axis (LA transverse diameter, LA superior-inferior dimension and LA anteroposterior diameter), and the center of mass (average radius [AR], AR = mean (A1, B1, A2, B2, A3, B3), where A1 + B1 = LA transverse diameter; A2 + B2 = LA superior-inferior dimension; and A3 + B3 = LA anteroposterior diameter divided by the center of mass of LA) (Figs. 2a–2c). In accordance with the formula proposed by Bisbal et al.,4 the coefficient of variation of the sphere (CVS = SDR/AR, where SDR = sd (A1, B1, A2, B2, A3, B3), sd—standard deviation) was obtained to define the LASP via the formula LASP = (1 − CVS) × 100. The interpretation of volume coefficient (VC) as an estimate of the volume of LA is explained by the fact that (2 × π/9) × VC is the mean of the volumes of spheres with the radii A1, B1, A2, B2, A3, B3.
Three planes of left atrium (LA) on computed tomography, based on the principal axis (LA transverse diameter, LA superior-inferior dimension and LA anteroposterior diameter), and the center of mass; A1 + B1 = LA transverse diameter (a); A2 + B2 = LA superior-inferior dimension (b); and A3 + B3 = LA anteroposterior diameter (c) divided by the center of mass of LA.
The discriminant function calculated by the R function lda is the following: 0.0161 × A1 − 0.0192 × A2 + 0.1076 × A3 − 0.0016 × B1 − 0.0694 × B2 + 0.1321 × B3 − 0.1102 × LASP + 0.0061 × VC/10000
Statistical Analysis
Data are presented as frequencies and percentages for categorical variables, and as means ± standard deviations (for approximately normally distributed variables), or medians and inter-quartile ranges (25–75th), (skewed distribution) for continuous variables. The χ2 or Fisher exact tests were used to compare proportions between the groups. Student's t-test and Welch's t-test were used to compare continuous variables between groups according to normality assumptions. Collinearity between variables was assessed using the Pearson correlation coefficient. Univariate analysis and multivariate logistic regression analysis were performed to identify factors predictive of stroke. Finally, receiver operator characteristic curves were constructed, and c-statistics (area under curve) were calculated to evaluate the value of specific factors for identification of patients with a history of ischemic stroke. A p value of ≤ 0.05 was considered significant. All analyses were conducted using R language and environment for statistical computing.12 For the discriminant analysis, the lda function available in the R package (library MASS) was used. The lda function enables a calculation of the probability of belonging either group, using the method of cross-validation.
Results
The study involved 157 patients: 74 in the stroke group (71.4 ± 11.0 years) and 83 in the control group (61.4 ± 10.1 years). Table 1 shows baseline patients characteristics.
Compared with the control group, the patients in the stroke group were older (p < 0.0001), had a higher CHA2DS2-VASc score (3.7 ± 1.7 vs. 1.60 ± 1.29, p < 0.0001) and HAS-BLED score (risk of bleeding complications) (3.0 ± 1.0 vs. 1.6 ± 1.3, p < 0.0001). Furthermore, the patients in the stroke group were more prone to comorbidities such as hypertension, congestive heart failure, and diabetes mellitus (p < 0.0001 for all). The patients in the stroke group had a greater LAA volume (12.2 ±7.6 vs. 10.0 ± 4.5 cm3, p = 0.0339) and LA (149.4 ± 95.5 vs. 124.1 ± 37.6 cm3; p = 0.0356). They also had a greater LA transverse diameter (77.0 ± 10.4 vs. 72.4 ± 6.4 mm; p = 0.0086) and LA anteroposterior diameter (9.2 ± 3.8 vs. 7.2 ± 2.9 mm; p = 0.002).
The mean heart rate during CT imaging was 73.80 in the stroke group and 64.99 in the control group (p value = 0.0117). In the 59 patients (80.82%) within the study group and 71 (91.03%) in the control group, CT scans were obtained during sinus rhythm (p value = 0.01174), in the remaining patients AF was recorded. (There are no data for 5 patients in the control group, and 1 patient in the study group).
In the univariate analysis, parameters significantly associated with previous stroke included diabetes mellitus, hypertension and congestive heart failure, CHA2DS2-VASc score, volume of LA and LAA, LA transverse and anteroposterior diameter as well as LA sphericity (Table 2).
Multivariate logistic regression analysis including demographic, clinical variables and LA and LAA morphological parameters, revealed that the variables associated with stroke were gender (OR 6.50; [1.03–41.03]; p = 0.0421), diabetes (OR 72.01; [4.77–1086.97]; p = 0.016), CHA2DS2-VASc score (OR 4.67; [2.09–10.40]; p = 0.0001), LA anteroposterior diameter (OR 1.24; [1.06–1.44]; p = 0.0050) and LA sphericity (OR 0.76; [0.66–0.87]; p = 0.0001) (Table 3).
Patients with prior stroke had lower LASP than those without (66.6 ± 10.3 vs. 70.5 ± 6.9%; p = 0.0062). The ROC curve, and corresponding area under the ROC curve (AUC) for the LASP, showed that higher values of the LASP correspond to a smaller proportion of patients with previous stroke (almost for the entire curve False-Positive Rate > True-Positive Rate, AUC 0.3974, 95% CI 0.3062–0.4887) (Fig. 3). The same trends can be seen in Fig. 4.
Patients with lower LASP had significantly more often previous TE compared to those with higher values (67.3 vs. 28.8 vs. 45.3% for the first, second and third tertile of LASP, respectively, p = 0.0377) (Fig. 5).
After limiting the analysis to geometrical variables derived from CT of LA the most accurate identification of patients with a history of ischemic stroke was achieved by the use of a function of six geometrical measurements of the LA (A1, B1, A2, B2, A3, B3), the sphericity coefficient and volume coefficient.
In ROC analysis, the AUC was 0.7273 (95% CI 0.6475–0.8071) for the above discriminant function for identification of patients with previous stroke. The cut-off value of (− 0.0863) had a sensitivity of 77% and a specificity of 65% in identifying patients with previous stroke (Fig. 6).
The addition of the discriminant function to the CHA2DS2-VASc score (calculated before stroke onset) increased the performance of the risk score alone: the AUC was 0.9004 and 0.9357, respectively (Fig. 7).
Patients receiving proper anticoagulation therapy as in-home medication (received novel oral anticoagulants at a standard dose, or a vitamin K antagonist while maintaining therapeutic INR levels on admission) before stroke onset have been additionally analyzed and compared with others in the stroke group. Anticoagulation had no impact on the results (Fig. 1S in Supplement Data).
Discussion
In this study, the association between the LA shape and the risk of stroke was found using a simple computation of the anatomical measurement of the LA based on CT imaging. The study showed the addition of LASP to CHA2DS2-VASc score might further improve identification of patients at risk of TE. Moreover, it was found that a function of six geometrical measurements, the sphericity coefficient and volume coefficient, had a greater ability than LASP alone to identify patients at risk of TE. The clinical use of this parameter may be particularly valuable in patients classified as low risk based on the CHA2DS2-VASc score system, in whom antithrombotic therapy is not recommended. The inclusion of the LA shape may lead to reclassification of risk, and therapy indication.
AF is an important factor associated with LA dilatation, and the association of LA enlargement and AF has been well recognized. The standard for linear LA measurement is the M-mode or 2-D derived anteroposterior linear dimension.8 However, this measurement may not reflect the volume of LA. The measurement of LA volume is currently recommended to assess the size of LA and remodeling. LA volume reflects long-term LA exposure to elevated left ventricular filling pressure. Therefore, the size of LA, especially expressed as its volume, is a valuable parameter enabling long-term hemodynamic monitoring of patients and is a strong predictor of outcomes.8 Although echocardiography is the most widely used method for evaluation of the LA, CT is emerging as a valuable method for assessing the shape and size of LA. 2D echocardiography significantly underestimates LA volumes, in comparison with cardiac CT.1 Previous studies have shown that some anatomical and functional LA and LAA features are independently associated with prevalent stroke in a population of patients with AF. The results of studies examining the relationship between the LASP and stroke are conflicting. In a recent study, three-dimensional LA shape assessed by means of LASP was identified as an independent predictor of recurrence after AF ablation and increased risk of TE.3, 4, 11
Nunes et al. showed that more spherical LA shape was independently associated with an increased risk for TE in a specific population with rheumatic mitral stenosis.10
Our findings differ from those reported by Bisbal et al.3 This discrepancy may be due to differences in statistical approach used in our study. We calculated the proportion of strokes in the three tertiles of LASP. Patients with lower LASP had significantly higher proportion of previous TE compared to those with higher values. We conducted a Wilcoxon-Mann-Whitney test to verify the hypothesis about the lack of differences between the stroke and control groups. The test rejects this hypothesis and favors the alternative hypothesis, saying that the LASP in the stroke group is lower (p = 0.0239). This confirms our previous findings that higher LASP is associated with a lower probability of stroke. Another reason for different results obtained by our study and Bisbal et al. is the fact that we studied a slightly older patient population. Furthermore, Bisbal et al. study was based on smaller sample size (only 29 patients in the study and control groups) and this was a highly selected, low-risk AF population (the study population presented a high proportion of low (CHA2DS2-VASc ≤ 1) or very low (CHA2DS2-VASc = 0) anticipated stroke risk (82.8 and 43.1%, respectively). The authors pointed out that LA imaging was not conducted at the time of TE, the sphericity and volume of LA change over time.4 In our study, CT imaging in the vast majority of patients in the study group was performed within one month of the onset of stroke during hospitalization in the neurorehabilitation ward.
Our study showed that presenting data in the form of six geometric measurements of the LA allowed for identification of patients with prior stroke with the probability of correct diagnosis of 70%. We presented a discriminant function of six geometrical measurements, the sphericity coefficient and volume coefficient which provided the most satisfactory quality of identification of patients with previous stroke, and had a better accuracy compared to the LASP. The probability of incorrect classification (PIC) using this function and for the optimal selection of the threshold was 0.3185. Because this result was obtained using the cross-validation (leave-one-out) method, this allowed us to conclude that 0.3185 is an approximately unbiased error estimate for stroke risk assessment for a new patient.
There are several potential limitations of our study. Firstly, although the study prospectively recruited patients with a history of ischemic stroke, data concerning their medication at the time of stroke has been derived from the patient files which might have influenced the accuracy of analysis. Secondly, we assumed that all strokes were due to thrombus migration from the LAA to the central nervous system, however, other causes of stroke could not be excluded. Finally, the study included patients who already suffered from stroke and all studied parameters were measured after the stroke. Thus, we may only examine an associated between these parameters and history of stroke whereas their true predictive value should be confirmed in larger prospective study including patients who are at risk of stroke but have not yet developed this complication.
In conclusion, LASP is an independent risk factor for TE in patients with AF. The patients with higher LASP had a significantly lower incidence of previous stroke compared to those with lower values. The proposed new formula for identification of AF patients who are at risk of stroke, based on six geometrical measurements of LA and the sphericity coefficient and volume coefficient, is superior to the LASP in identification of AF patients with a history of ischemic stroke.
References
Avelar, E., R. Durst, G. A. Rosito, M. Thangaroopan, et al. Comparison of the accuracy of multidetector computed tomography versus two-dimensional echocardiography to measure left atrial volume. Am. J. Cardiol. 106:104–109, 2010. https://doi.org/10.1016/j.amjcard.2010.02.021.
Baran, J., I. Michałowska, I. Kowalik, and P. Kułakowski. assessment of the left atrial appendage morphology in patients after ischemic stroke—the ASSAM study protocol. Kardiol. Polska. 75:758–762, 2017.
Bisbal, F., F. Gómez-Pulido, P. Cabanas-Grandío, et al. left atrial geometry improves risk prediction of thromboembolic events in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 27:804–810, 2016.
Bisbal, F., E. Guiu, N. Calvo, et al. Left atrial sphericity: a new method to assess atrial remodeling Impact on the outcome of atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 24:752–759, 2013.
Fang, M. C., A. S. Go, Y. Chang, L. Borowsky, et al. Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J. Am. Coll. Cardiol. 51:810–815, 2008.
Ferro, J. M. Cardioembolic stroke: an update. Lancet Neurol. 2(3):177–188, 2003. https://doi.org/10.1016/s1474-4422(03)00324-7.
Kirchhof, P., S. Benussi, D. Kotecha, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 37:2893–2962, 2016.
Lang, R. M., L. P. Badano, V. Mor-Avi, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28(1):1-39.e14, 2015. https://doi.org/10.1016/j.echo.2014.10.003.
Mozaffarian, D., E. J. Benjamin, A. S. Go, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 133:447–454, 2016.
Nunes, M. C., M. D. Handschumacher, R. A. Levine, et al. Role of LA shape in predicting embolic cerebrovascular events in mitral stenosis: mechanistic insights from 3D echocardiography. JACC Cardiovasc. Imaging. 7:453–461, 2014.
Oliver, W., G. Matthews, C. R. Ayers, et al. Factors associated with left atrial remodeling in the general population. Circ. Cardiovasc. Imaging. 10(2):e005047, 2017.
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, 2016. https://www.R-project.org/.
Van Staa, T. P., E. Setakis, G. L. Di Tanna, et al. A comparison of risk stratification schemes for stroke in 79,884 atrial fibrillation patients in general practice. J. Thromb. Haemost. 9:39–48, 2011.
Wolf, P. A., R. D. Abbott, and W. B. Kannel. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 22:983–988, 1991.
Conflict of interest
Authors declare no conflicts of interest.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Funding
This work was supported by a research grant from the Centre of Postgraduate Medical Education (Grant No. 501-1-10-14-15).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Igor Efimov oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13239_2021_587_MOESM1_ESM.doc
Fig. 1S Scatter plot of points belonging to three classes in the plane of canonical variables. Discrimination based on the left atrium geometry of 3 groups (1 – control group, 2 – stroke group without anticoagulant treatment, 3 – stroke group proper anticoagulation therapy). LD1 – first canonical variable, LD2 – second canonical variable (DOC 47 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dudzińska-Szczerba, K., Zalewska, M., Niemiro, W. et al. Association of Left Atrial Sphericity with Risk of Stroke in Patients with Atrial Fibrillation. Sub-Analysis of the ASSAM Study. Cardiovasc Eng Tech 13, 419–427 (2022). https://doi.org/10.1007/s13239-021-00587-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-021-00587-y