Abstract
Purpose
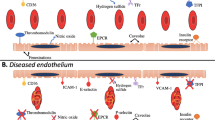
Endothelial cell (EC) dysfunction underlies the pathology of multiple disease conditions including cardiovascular and pulmonary diseases. Dysfunctional ECs have a distinctive gene expression profile compared to healthy ECs. RNAi therapy is a powerful therapeutic approach that can be used to silence multiple genes of interests simultaneously. However, the delivery of RNAi to ECs in vivo continues to be a major challenge. Here, we optimized a polymer formulation based on poly(β-amino ester)s (pBAEs) to deliver siRNA to vascular ECs.
Methods
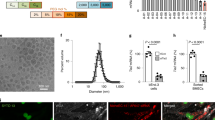
We developed a library of bioinspired oligopeptide-modified pBAE nanoparticles (NPs) with different physicochemical proprieties and screened them for cellular uptake and efficacy of RNAi delivery in vitro using ECs, vascular smooth muscle cells, and THP-1 monocytes. From the screening, the lysine-/histidine-oligopeptide modified pBAE (C6-KH) NP was selected and further tested ex vivo using mouse aorta and in mice to determine efficiency of siRNA delivery in vivo.
Results
The in vitro screening study showed that C6-KH was most efficient in delivering siRNA to ECs. Ex vivo study showed that C6-KH nanoparticles containing siRNAs accumulated in the endothelial layer of mouse aortas. In vivo study showed that C6-KH nanoparticles carrying siICAM2 injected via tail-vein in mice significantly reduced ICAM2 level in the artery endothelium (55%), lung (52%), and kidney (31%), but not in the liver, heart, and thymus, indicating a tissue-specific delivery pattern.
Conclusions
We demonstrate that C6-KH pBAE can used for delivery of siRNAs to the artery endothelium and lung, while minimizing potential side or toxic effects in the liver and heart.






Similar content being viewed by others
References
Behr, J. The proton sponge: a trick to enter cells the viruses did not exploit. Chimia 2:34–36, 1997.
Dahlman, J. E., C. Barnes, O. F. Khan, A. Thiriot, S. Jhunjunwala, T. E. Shaw, Y. Xing, H. B. Sager, G. Sahay, L. Speciner, A. Bader, R. L. Bogorad, H. Yin, T. Racie, Y. Dong, S. Jiang, D. Seedorf, A. Dave, K. Singh Sandhu, M. J. Webber, T. Novobrantseva, V. M. Ruda, A. K. R. R. Lytton-Jean, C. G. Levins, B. Kalish, D. K. Mudge, M. Perez, L. Abezgauz, P. Dutta, L. Smith, K. Charisse, M. W. Kieran, K. Fitzgerald, M. Nahrendorf, D. Danino, R. M. Tuder, U. H. Von Andrian, A. Akinc, D. Panigrahy, A. Schroeder, V. Koteliansky, R. Langer, and D. G. Anderson. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat. Nanotechnol. 9:648–655, 2014. https://doi.org/10.1038/nnano.2014.84.
Dai, G., M. R. Kaazempur-Mofrad, S. Natarajan, Y. Zhang, S. Vaughn, B. R. Blackman, R. D. Kamm, G. Garcia-Cardena, and M. A. Gimbrone. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc. Natl. Acad. Sci. 101:14871–14876, 2004. https://doi.org/10.1073/pnas.0406073101.
Dosta, P., V. Ramos, and S. Borrós. Stable and efficient generation of poly(β-amino ester)s for RNAi delivery. Mol. Syst. Des. Eng. 3:677–689, 2018. https://doi.org/10.1039/C8ME00006A.
Dosta, P., N. Segovia, A. Cascante, V. Ramos, and S. Borrós. Surface charge tunability as a powerful strategy to control electrostatic interaction for high efficiency silencing, using tailored oligopeptide-modified poly(beta-amino ester)s (PBAEs). Acta Biomater. 20:82–93, 2015. https://doi.org/10.1016/j.actbio.2015.03.029.
Fornaguera, C., M. Guerra-Rebollo, M. Ángel Lázaro, C. Castells-Sala, O. Meca-Cortés, V. Ramos-Pérez, A. Cascante, N. Rubio, J. Blanco, and S. Borrós. mRNA delivery system for targeting antigen-presenting cells in vivo. Adv. Healthc. Mater. 7:1800335, 2018. https://doi.org/10.1002/adhm.201800335.
Fornaguera, C., M. Guerra-Rebollo, M. Á. Lázaro, A. Cascante, N. Rubio, J. Blanco, and S. Borrós. In vivo retargeting of poly(beta aminoester) (OM-PBAE) nanoparticles is influenced by protein Corona. Adv. Healthc. Mater. 8:1900849, 2019. https://doi.org/10.1002/adhm.201900849.
Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 7:5577, 2012. https://doi.org/10.2147/IJN.S36111.
Green, J. J., R. Langer, and D. G. Anderson. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res. 41:749–759, 2008. https://doi.org/10.1021/ar7002336.
Hajj, K. A., J. R. Melamed, N. Chaudhary, N. G. Lamson, R. L. Ball, S. S. Yerneni, and K. A. Whitehead. A potent branched-tail lipid nanoparticle enables multiplexed mRNA delivery and gene editing in vivo. Nano Lett. 2020. https://doi.org/10.1021/acs.nanolett.0c00596.
Heath, J. M., J. Fernandez Esmerats, L. Khambouneheuang, S. Kumar, R. Simmons, and H. Jo. Mechanosensitive microRNA-181b regulates aortic valve endothelial matrix degradation by targeting TIMP3. Cardiovasc. Eng. Technol. 9:141–150, 2018. https://doi.org/10.1007/s13239-017-0296-z.
Kanasty, R., J. R. Dorkin, A. Vegas, and D. Anderson. Delivery materials for siRNA therapeutics. Nat. Mater. 12:967–977, 2013. https://doi.org/10.1038/nmat3765.
Kaufmann, J., K. Ahrens, and A. Santel. RNA interference for therapy in the vascular endothelium. Microvasc. Res. 80:286–293, 2010. https://doi.org/10.1016/j.mvr.2010.02.002.
Knudsen, K. B., H. Northeved, P. E. K. Kumar, A. Permin, T. Gjetting, T. L. Andresen, S. Larsen, K. M. Wegener, J. Lykkesfeldt, K. Jantzen, S. Loft, P. Møller, and M. Roursgaard. In vivo toxicity of cationic micelles and liposomes. Nanomed. Nanotechnol. Biol. Med. 11:467–477, 2015. https://doi.org/10.1016/j.nano.2014.08.004.
Kumar, S., D. Williams, S. Sur, J.-Y. Wang, and H. Jo. Role of flow-sensitive microRNAs and long noncoding RNAs in vascular dysfunction and atherosclerosis. Vascul. Pharmacol. 114:76–92, 2019. https://doi.org/10.1016/j.vph.2018.10.001.
Lv, H., S. Zhang, B. Wang, S. Cui, and J. Yan. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release. 114:100–109, 2006. https://doi.org/10.1016/j.jconrel.2006.04.014.
Lynn, D. M., and R. Langer. Degradable poly(β-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J. Am. Chem. Soc. 122:10761–10768, 2000. https://doi.org/10.1021/ja0015388.
Miao, L., J. Lin, Y. Huang, L. Li, D. Delcassian, Y. Ge, Y. Shi, and D. G. Anderson. Synergistic lipid compositions for albumin receptor mediated delivery of mRNA to the liver. Nat. Commun. 11:2424, 2020. https://doi.org/10.1038/s41467-020-16248-y.
Nam, D., C.-W. Ni, A. Rezvan, J. Suo, K. Budzyn, A. Llanos, D. Harrison, D. Giddens, and H. Jo. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am. J. Physiol. Circ. Physiol. 297:H1535–H1543, 2009. https://doi.org/10.1152/ajpheart.00510.2009.
Ni, C.-W., S. Kumar, C. J. Ankeny, and H. Jo. Development of immortalized mouse aortic endothelial cell lines. Vasc. Cell 6:7, 2014. https://doi.org/10.1186/2045-824X-6-7.
Núñez-Toldrà, R., P. Dosta, S. Montori, V. Ramos, M. Atari, and S. Borrós. Improvement of osteogenesis in dental pulp pluripotent-like stem cells by oligopeptide-modified poly(β-amino ester)s. Acta Biomater. 53:152–164, 2017. https://doi.org/10.1016/j.actbio.2017.01.077.
Pober, J. S., and W. C. Sessa. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 7:803–815, 2007. https://doi.org/10.1038/nri2171.
Pober, J. S., and W. C. Sessa. Inflammation and the blood microvascular system. Cold Spring Harb. Perspect. Biol. 7:a016345, 2015. https://doi.org/10.1101/cshperspect.a016345.
Santel, A., M. Aleku, O. Keil, J. Endruschat, V. Esche, B. Durieux, K. Löffler, M. Fechtner, T. Röhl, G. Fisch, S. Dames, W. Arnold, K. Giese, A. Klippel, and J. Kaufmann. RNA interference in the mouse vascular endothelium by systemic administration of siRNA-lipoplexes for cancer therapy. Gene Ther. 13:1360–1370, 2006. https://doi.org/10.1038/sj.gt.3302778.
Santel, A., M. Aleku, O. Keil, J. Endruschat, V. Esche, G. Fisch, S. Dames, K. Löffler, M. Fechtner, W. Arnold, K. Giese, A. Klippel, and J. Kaufmann. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 13:1222–1234, 2006. https://doi.org/10.1038/sj.gt.3302777.
Segovia, N., P. Dosta, A. Cascante, V. Ramos, and S. Borrós. Oligopeptide-terminated poly(β-amino ester)s for highly efficient gene delivery and intracellular localization. Acta Biomater. 10:2147–2158, 2014. https://doi.org/10.1016/j.actbio.2013.12.054.
Semple, S. C., A. Akinc, J. Chen, A. P. Sandhu, B. L. Mui, C. K. Cho, D. W. Y. Sah, D. Stebbing, E. J. Crosley, E. Yaworski, I. M. Hafez, J. R. Dorkin, J. Qin, K. Lam, K. G. Rajeev, K. F. Wong, L. B. Jeffs, L. Nechev, M. L. Eisenhardt, M. Jayaraman, M. Kazem, M. A. Maier, M. Srinivasulu, M. J. Weinstein, Q. Chen, R. Alvarez, S. A. Barros, S. De, S. K. Klimuk, T. Borland, V. Kosovrasti, W. L. Cantley, Y. K. Tam, M. Manoharan, M. A. Ciufolini, M. A. Tracy, A. de Fougerolles, I. MacLachlan, P. R. Cullis, T. D. Madden, and M. J. Hope. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 28:172–176, 2010. https://doi.org/10.1038/nbt.1602.
Setten, R. L., J. J. Rossi, and S. Han. The current state and future directions of RNAi-based therapeutics. Nat. Rev. Drug Discov. 18:421–446, 2019. https://doi.org/10.1038/s41573-019-0017-4.
Son, D. J., S. Kumar, W. Takabe, C. WooKim, C.-W. Ni, N. Alberts-Grill, I.-H. Jang, S. Kim, W. Kim, S. WonKang, A. H. Baker, J. WoongSeo, K. W. Ferrara, and H. Jo. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat. Commun. 4:3000, 2013. https://doi.org/10.1038/ncomms4000.
Soutschek, J., A. Akinc, B. Bramlage, K. Charisse, R. Constien, M. Donoghue, S. Elbashir, A. Geick, P. Hadwiger, J. Harborth, M. John, V. Kesavan, G. Lavine, R. K. Pandey, T. Racie, K. G. Rajeev, I. Röhl, I. Toudjarska, G. Wang, S. Wuschko, D. Bumcrot, V. Koteliansky, S. Limmer, M. Manoharan, and H.-P. Vornlocher. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 432:173–178, 2004. https://doi.org/10.1038/nature03121.
Tang, F., and T.-L. Yang. MicroRNA-126 alleviates endothelial cells injury in atherosclerosis by restoring autophagic flux via inhibiting of PI3K/Akt/mTOR pathway. Biochem. Biophys. Res. Commun. 495:1482–1489, 2018. https://doi.org/10.1016/j.bbrc.2017.12.001.
Vaishnaw, A. K., J. Gollob, C. Gamba-Vitalo, R. Hutabarat, D. Sah, R. Meyers, T. de Fougerolles, and J. Maraganore. A status report on RNAi therapeutics. Silence. 1:14, 2010. https://doi.org/10.1186/1758-907X-1-14.
Zampetaki, A., S. Kiechl, I. Drozdov, P. Willeit, U. Mayr, M. Prokopi, A. Mayr, S. Weger, F. Oberhollenzer, E. Bonora, A. Shah, J. Willeit, and M. Mayr. Plasma MicroRNA profiling reveals loss of endothelial MiR-126 and other MicroRNAs in Type 2 diabetes. Circ. Res. 107:810–817, 2010. https://doi.org/10.1161/CIRCRESAHA.110.226357.
Zugates, G. T., N. C. Tedford, A. Zumbuehl, S. Jhunjhunwala, C. S. Kang, L. G. Griffith, D. Lauffenburger, R. Langer, and D. G. Anderson. Gene Delivery properties of end-modified poly(β-amino ester)s. Bioconjug. Chem. 18:1887–1896, 2007. https://doi.org/10.1021/bc7002082.
Author Contributions
PD: Conception and design, Collection and assembly of data, Data analysis and interpretation, Manuscript writing. CD: Data analysis and interpretation, Manuscript writing. VR: Conception and design Data analysis and interpretation, Manuscript writing, Final approval of manuscript. SK: Collection of data. DWK: Collection of data. HJ: Conception and design, Data analysis and interpretation, Manuscript writing, Final approval of manuscript. SB: Conception and design, Data analysis and interpretation, Manuscript writing, Final approval of manuscript.
Funding
This work was supported by funding from the National Institutes of Health grants HL119798 and HL095070 to HJ. It was also supported by funding from the Spanish Ministerio de Ciencia, Innovación y Universidades for the Grant RTI2018-094734-B-C22 to SB. PD received the financial support from AGAUR (Generalitat de Catalunya) 2017FI_B2 00141. HJ was supported by John and Jan Portman Professorship and Wallace H. Coulter Distinguished Faculty Professorship. This study was also funded by Grup d’Enginyeria dels Materials (GEMAT). GEMAT would like to acknowledge Agència de Gestió d’ajuts Universitaris i de Recerca, Generalitat de Catalunya (SGR 2017) nº 1559.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Keefe B. Manning oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dosta, P., Demos, C., Ramos, V. et al. Delivery of siRNA to Endothelial Cells In Vivo Using Lysine/Histidine Oligopeptide-Modified Poly(β-amino ester) Nanoparticles. Cardiovasc Eng Tech 12, 114–125 (2021). https://doi.org/10.1007/s13239-021-00518-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-021-00518-x