Abstract
Introduction
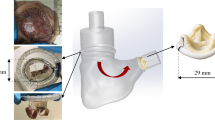
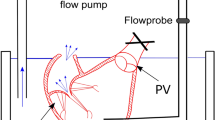
Tricuspid regurgitation (TR) affects approximately 1.6 million Americans and is associated with just a 63.9% 1-year survival rate in its moderate to severe forms due to its asymptomatic nature and late diagnosis and surgical referral. As a result, industrial fervor has begun to broach this topic, with several percutaneous treatment devices currently under development. As much remains unknown about the tricuspid apparatus, the mechanics of these procedures remain unquantified. In this study, a testing apparatus and technique for the evaluation of percutaneous tricuspid valve (TV) bicuspidization were developed for the evaluation of these parameters in twelve porcine hearts.
Methods
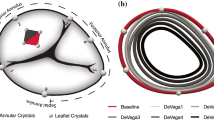
The passive relaxed myocardial state and the active contracted state were each induced in six porcine hearts and the bicuspidization experiment was run twice, the second time after induction of TR. TV annular area, cinching force, static leakage through the TV annulus, and annular ellipticity were quantified and compared among the groups.
Results
The use of phenol was effective to induce functional TR by increased annular area. Cinching force was not found to differ between any of the testing states, but the bicuspidization experiment was able to reduce the TR annular area to that of its healthy counterpart in addition to reducing static leakage through the TV annulus. Despite appropriately reducing the area, bicuspidization was found to induce a more circular TV annular shape.
Conclusion
Taken together, these results provide a first mechanical analysis of the TV bicuspidization mechanism and may serve as a point of reference for future clinical animal studies.







Similar content being viewed by others
References
Adkins, A., et al. Force required to cinch the tricuspid annulus: an ex-vivo study. J. Heart Valve Dis. 24(5):644, 2015.
Aleman, J., et al. Effects of Cinching Force on the Tricuspid Annulus: A Species Comparison. J. Cardiovasc. Dis. Diagn. 5:4, 2017.
Amini Khoiy, K., et al. Surface strains of porcine tricuspid valve septal leaflets measured in ex vivo beating hearts. J. Biomech. Eng. 138(11):111006, 2016.
Besler, C., C. U. Meduri, and P. Lurz. Transcatheter treatment of functional tricuspid regurgitation using the trialign device. Interv. Cardiol. Rev. 13(1):8, 2018.
Bhattacharya, S., et al. Tension to passively cinch the mitral annulus through coronary sinus access: An ex vivo study in ovine model. J. Biomech. 47(6):1382–1388, 2014.
Cilingiroglu, M., and K. Marmagkiolis. Percutanous management of tricuspid regurgitation: The “Achilles tendon” of transcatheter valve interventions. Catheterization Cardiovasc. Interv. 88(2):294–295, 2016.
Colombo, T., et al. Tricuspid regurgitation secondary to mitral valve disease: tricuspid annulus function as guide to tricuspid valve repair. Cardiovasc. Surg. 9(4):369–377, 2001.
Correia, P., G. Coutinho, and M. Antunes. Tricuspid valve: a valve not to be forgotten. E-J. Cardol. Pract. 11:20, 2013.
Di Mauro, M., et al. Mitral valve surgery for functional mitral regurgitation: prognostic role of tricuspid regurgitation☆. Eur. J. Cardio-Thorac. Surg. 35(4):635–640, 2009.
Dreyfus, G. D., et al. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann. Thorac. Surg. 79(1):127–132, 2005.
Fender, E. A., R. A. Nishimura, and D. R. Holmes. Percutaneous therapies for tricuspid regurgitation. Expert review of medical devices 14(1):37–48, 2017.
Fukuda, S., et al. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: a real-time, 3-dimensional echocardiographic study. Circulation 114(1_supplement):I-492-I–498, 2006.
Ghanta, R. K., et al. Suture bicuspidization of the tricuspid valve versus ring annuloplasty for repair of functional tricuspid regurgitation: Midterm results of 237 consecutive patients. J Thorac. Cardiovasc. Surg. 133(1):117–126, 2007.
Giannini, F. and Latib, A. (2016) The Next Frontier of Percutaneous Tricuspid Valve Repair.
Granegger, M., et al. A passive beating heart setup for interventional cardiology training. Curr. Dir. Biomed. Eng. 2(1):735–739, 2016.
Green, G. R., et al. Mitral annular dilatation and papillary muscle dislocation without mitral regurgitation in sheep. Circulation 100(2):II-95-II-102, 1999.
Hahn, R. T., et al. Early feasibility study of a transcatheter tricuspid valve annuloplasty: SCOUT trial 30-day results. J. Am. Coll. Cardiol. 69(14):1795–1806, 2017.
Hahn, R. T., et al. Early feasibility study of a transcatheter tricuspid valve annuloplasty: SCOUT Trial 30-day results. J. Am. Coll. Cardiol. 69(14):1795–1806, 2017.
Hung, J. The pathogenesis of functional tricuspid regurgitation. Semin. Thorac. Cardiovasc. Surg. 22(1):76–78, 2010.
Huntington, A., et al. Anisotropy of the Passive and Active Rat Vagina Under Biaxial Loading. Ann. Biomed. Eng. 47(1):272–281, 2019.
Jaworek, M., et al. Long-arm Clip for Transcatheter Edge-to-Edge Treatment of Mitral and Tricuspid Regurgitation–Ex-Vivo Beating Heart Study. Struct. Heart 3(3):211–219, 2019.
Jeong, D. S., et al. Fate of functional tricuspid regurgitation in aortic stenosis after aortic valve replacement. J. Thorac. Cardiovasc. Surg. 148(4):1328–1333, 2014.
Kato, T., et al. Inhibitory effects and active constituents of Alisma rhizomes on vascular contraction induced by high concentration of KCl. Bull. Chem. Soc. Japan 67(5):1394–1398, 1994.
Kay, J. H., G. Maselli-Campagna, and H. K. Tsuji. Surgical treatment of tricuspid insufficiency. Ann. Surg. 162(1):53, 1965.
Khoiy, K. A., and R. Amini. On the biaxial mechanical response of porcine tricuspid valve leaflets. J. Biomech. Eng. 138(10):104504, 2016.
Kim, Y.-J., et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation 120(17):1672–1678, 2009.
Kragsnaes, E. S., et al. In-plane tricuspid valve force measurements: development of a strain gauge instrumented annuloplasty ring. Cardiovas. Eng. Technol. 4(2):131–138, 2013.
Kuwaki, K., et al. Tricuspid valve surgery for functional tricuspid valve regurgitation associated with left-sided valvular disease. Eur. J. Cardio-Thorac. Surg. 20(3):577–582, 2001.
Latib, A., et al. Percutaneous bicuspidization of the tricuspid valve. JACC 10(4):488–489, 2017.
Leopaldi, A., et al. A novel passive left heart platform for device testing and research. Med. Eng. Phys. 37(4):361–366, 2015.
Li, R.-K., et al. Autologous porcine heart cell transplantation improved heart function after a myocardial infarction. J. Thorac. Cardiovasc. Surg. 119(1):62–68, 2000.
Lurz, P., et al. Early experience of the trialign system for catheter-based treatment of severe tricuspid regurgitation. Eur. Heart J. 37(47):3543–3543, 2016.
Madukauwa-David, I. D., et al. Suture dehiscence and collagen content in the human mitral and tricuspid annuli. Biomech. Model. Mechanobiol. 8(2):291–299, 2018.
Madukauwa-David, I. D., et al. Suture dehiscence and collagen content in the human mitral and tricuspid annuli. Biomech. Model. Mechanobiol. 18(2):291–299, 2019.
Malinowski, M., et al. Tricuspid annular geometry and strain after suture annuloplasty in acute ovine right Heart failure. Ann. Thorac. Surg. 106(6):1804–1811, 2018.
Malinowski, M., et al. Impact of tricuspid annular size reduction on right ventricular function, geometry and strain. Eur. J. Cardio-Thorac. Surg. 2019. https://doi.org/10.1093/ejcts/ezy484.
Malinowski, M., et al. Sonomicrometry-derived 3-dimensional geometry of the human tricuspid annulus. J. Thorac. Cardiovasc. Surg. 157(4):1452–1461, 2019.
Marciniak, A., K. Glover, and R. Sharma. Cohort profile: prevalence of valvular heart disease in community patients with suspected heart failure in UK. BMJ open 7(1):e012240, 2017.
McCarthy, P. M., et al. Tricuspid valve repair: durability and risk factors for failure. The Journal of Thoracic and Cardiovascular Surgery 127(3):674–685, 2004.
Nath, J., E. Foster, and P. A. Heidenreich. Impact of tricuspid regurgitation on long-term survival. J. Am. Coll. Cardiol. 43(3):405–409, 2004.
Nijenhuis, V., et al. The last frontier: transcatheter devices for percutaneous or minimally invasive treatment of chronic heart failure. Netherlands Heart J. 25(10):536–544, 2017.
Nishimura, R. A., et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129(23):e521–643, 2014.
Nkomo, V. T., et al. Burden of valvular heart diseases: a population-based study. Lancet 368(9540):1005–1011, 2006.
Paul, D. M., et al. Suture dehiscence in the tricuspid annulus: an ex vivo analysis of tissue strength and composition. Ann. Thorac. Surg. 104(3):820–826, 2017.
Pierce, E. L., et al. How local annular force and collagen density govern mitral annuloplasty ring dehiscence risk. Ann. Thorac. Surg. 102(2):518–526, 2016.
Pokutta-Paskaleva, A., et al. Comparative mechanical, morphological, and microstructural characterization of porcine mitral and tricuspid leaflets and chordae tendineae. Acta Biomater. 85:241–252, 2019.
Rausch, M. K., et al. Engineering analysis of tricuspid annular dynamics in the beating ovine heart. Ann. Biomed. Eng. 46(3):443–451, 2018.
Rausch, M. K., et al. The effect of acute pulmonary hypertension on tricuspid annular height, strain, and curvature in sheep. Cardiovasc. Eng. Technol. 9(3):365–376, 2018.
Rodés-Cabau, J., et al. Transcatheter therapies for treating tricuspid regurgitation. Journal of the American College of Cardiology 67(15):1829–1845, 2016.
Schofer, J., et al. First-in-human transcatheter tricuspid valve repair in a patient with severely regurgitant tricuspid valve. J. Am. Coll. Cardiol. 65(12):1190–1195, 2015.
Singh, J. P., et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am. J. Cardiol. 83(6):897–902, 1999.
Spinner, E. M., et al. In vitro characterization of the mechanisms responsible for functional tricuspid regurgitation. Circulation 124(8):920–929, 2011.
Stuge, O., and J. Liddicoat. Emerging opportunities for cardiac surgeons within structural heart disease. J. Thorac. Cardiovasc. Surg. 132(6):1258–1261, 2006.
Stuge, O. and J. Liddicoat, Emerging opportunities for cardiac surgeons within structural heart disease. 2006, Mosby.
Taramasso, M., et al. Percutaneous tricuspid valve therapies: the new frontier. European heart journal 38(9):639–647, 2017.
Ton-Nu, T.-T., et al. CLINICAL PERSPECTIVE. Circulation 114(2):143–149, 2006.
Tricuspid Regurgitation Global Strategic Market Assessmen, D. Consulting, Editor. 2016.
Tritthart, H., et al. Effects of Ca-free and EDTA-containing Tyrode solution on transmembrane electrical activity and contraction in guinea pig papillary muscle. Pflügers Archiv 338(4):361–376, 1973.
Van de Veire, N. R., et al. Tricuspid annuloplasty prevents right ventricular dilatation and progression of tricuspid regurgitation in patients with tricuspid annular dilatation undergoing mitral valve repair. J. Thorac. Cardiovasc. Surg. 141(6):1431–1439, 2011.
Vismara, R., et al. Transcatheter edge-to-edge treatment of functional tricuspid regurgitation in an ex vivo pulsatile heart model. J. Am. Coll. Cardiol. 68(10):1024–1033, 2016.
Acknowledgments
Fatiesa Sulejmani is also supported by the Georgia Institute of Technology-Emory University-Peking University Global Biomedical Engineering Research and Education Fellowship, as well as the Friends of Bobby Jones Fund from Emory University’s Laney Graduate School.
Conflict of interest
Dr. Wei Sun is a co-founder and serves as the Chief Scientific Advisor of Dura Biotech. He has received compensation and owns equity in the company. Fatiesa Sulejmani and Joshua Pataky declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Changfu Wu oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sulejmani, F., Pataky, J. & Sun, W. Mechanical and Structural Evaluation of Tricuspid Bicuspidization in a Porcine Model. Cardiovasc Eng Tech 11, 522–531 (2020). https://doi.org/10.1007/s13239-020-00480-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-020-00480-0