Abstract
Purpose
The objective of this study was to bioengineer 3D patches from cardiac myocytes that have been reprogrammed from human adipogenic mesenchymal stem cells (hADMSCs).
Methods
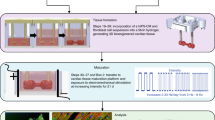
Human adipogenic mesenchymal stem cells (hADMSCs) were reprogrammed to form cardiac myocytes using transcription factors ETS2 and MESP1. Reprogrammed cardiac myocytes were cultured in a fibrin gel to bioengineer 3D patch patches. The effect of initial plating density (1–25 million cells per patch), time (28-day culture period) and treatment with 1 μM isoproterenol and 1 μM epinephrine were evaluated.
Results
3D patches were fabricated using cardiac myocytes that have been reprogrammed from hADMSCs. Based on optimization studies, it was determined that 10 million cells were needed to bioengineer a single patch, that measured 2 × 2 cm2. Furthermore, 3D patches fabricated 10 million cells were stable in culture for up to 28 days. Treatment of 3D patches with 1 μM isoproterenol and 1 μM epinephrine resulted in an increase in the electrical properties, as measured by electrical impulse amplitude and frequency. An increase in the expression of mTOR, KCNV1, GJA5, KCNJ16, CTNNT2, KCNV2, MYO3, FOXO1 and KCND2 was noted in response to treatment of 3D patches with isoproterenol and epinephrine.
Conclusion
Based on the results of this study, there is evidence to support the successful fabrication of a highly functional 3D patches with measurable electrical activity using cardiac myocytes reprogrammed from hADMSCs. 3D patches fabricated using optimal conductions described in this study can be used to improve the functional properties of failing hearts. Predominantly, in case of the infarcted hearts with partial loss of electrical activity, the electrical properties of the 3D patches may restore the electrical activity of the heart.







Similar content being viewed by others
References
Barile, L., F. Cerisoli, G. Frati, R. Gaetani, I. Chimenti, E. Forte, L. Cassinelli, L. Spinardi, C. Altomare, E. Kizana, A. Giacomello, E. Messina, S. Ottolenghi, and M. C. Magli. Bone marrow-derived cells can acquire cardiac stem cells properties in damaged heart. J. Cell Mol. Med. 15:63–71, 2011.
Blan, N. R., and R. K. Birla. Design and fabrication of heart muscle using scaffold-based tissue engineering. J. Biomed. Mater. Res. A 86:195–208, 2008.
Fernandes, S., A. V. Naumova, W. Z. Zhu, M. A. Laflamme, J. Gold, and C. E. Murry. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J. Mol. Cell. Cardiol. 49:941–949, 2010.
Hogan, M., M. Mohamed, Z. W. Tao, L. Gutierrez, and R. Birla. Establishing the framework to support bioartificial heart fabrication using fibrin-based three-dimensional artificial heart muscle. Artif. Organs 39:165–171, 2015.
Huang, Y. C., L. Khait, and R. K. Birla. Modulating the functional performance of bioengineered heart muscle using growth factor stimulation. Ann. Biomed. Eng. 36:1372–1382, 2008.
Inagawa, K., and M. Ieda. Direct reprogramming of mouse fibroblasts into cardiac myocytes. J. Cardiovasc. Transl. Res. 6:37–45, 2013.
Khait, L., and R. K. Birla. Effect of thyroid hormone on the contractility of self organized heart muscle. Vitro Cell Dev. Biol. Anim. 44:204–213, 2008.
Khait, L., and R. K. Birla. Bypassing the patient: comparison of biocompatible models for the future of vascular tissue engineering. Cell Transplant. 21:269–283, 2012.
Khait, L., L. Hecker, N. R. Blan, G. Coyan, F. Migneco, Y. C. Huang, and R. K. Birla. Getting to the heart of tissue engineering. J. Cardiovasc. Transl. Res. 1:71–84, 2008.
Khait, L., C. J. Hodonsky, and R. K. Birla. Variable optimization for the formation of three-dimensional self-organized heart muscle. Vitro Cell Dev. Biol. Anim. 45:592–601, 2009.
Li, M., M. Chen, W. Han, and X. Fu. How far are induced pluripotent stem cells from the clinic? Ageing Res. Rev. 9:257–264, 2010.
Ott, H. C., T. S. Matthiesen, S. K. Goh, L. D. Black, S. M. Kren, T. I. Netoff, and D. A. Taylor. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 14:213–221, 2008.
Patel, N. M., Z. W. Tao, M. A. Mohamed, M. K. Hogan, L. Gutierrez, and R. K. Birla. Engineering 3D bio-artificial heart muscle: the acellular ventricular extracellular matrix model. ASAIO J. 61:61–70, 2015.
Roger, V. L., A. S. Go, D. M. Lloyd-Jones, E. J. Benjamin, J. D. Berry, W. B. Borden, D. M. Bravata, S. Dai, E. S. Ford, C. S. Fox, H. J. Fullerton, C. Gillespie, S. M. Hailpern, J. A. Heit, V. J. Howard, B. M. Kissela, S. J. Kittner, D. T. Lackland, J. H. Lichtman, L. D. Lisabeth, D. M. Makuc, G. M. Marcus, A. Marelli, D. B. Matchar, C. S. Moy, D. Mozaffarian, M. E. Mussolino, G. Nichol, N. P. Paynter, E. Z. Soliman, P. D. Sorlie, N. Sotoodehnia, T. N. Turan, S. S. Virani, N. D. Wong, D. Woo, and M. B. Turner. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 125:e2–e220, 2012.
Tao, Z. W., M. Mohamed, M. Hogan, L. Gutierrez, and R. K. Birla. Optimizing a spontaneously contracting heart tissue patch with rat neonatal cardiac cells on fibrin gel. J. Tissue Eng. Regen. Med. 11:153–163, 2014.
Vazin, T., and W. J. Freed. Human embryonic stem cells: derivation, culture, and differentiation: a review. Restor. Neurol. Neurosci. 28:589–603, 2010.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Ajit Yoganathan oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbasgholizadeh, R., Islas, J.F., Navran, S. et al. A Highly Conductive 3D Cardiac Patch Fabricated Using Cardiac Myocytes Reprogrammed from Human Adipogenic Mesenchymal Stem Cells. Cardiovasc Eng Tech 11, 205–218 (2020). https://doi.org/10.1007/s13239-019-00451-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13239-019-00451-0