Abstract
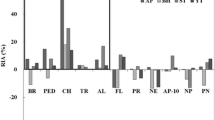
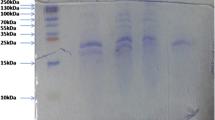
The present study describes the characterization of crude protease extract from zebra blenny (Salaria basilisca) and its evaluation in liquid detergent and shrimp waste deproteinization. At least five caseinolytic proteases clear bands were observed in zymogram. The crude alkaline protease showed optimum activity at pH 8.0 and 60 °C, and it was highly stable over a wide range of pH from 6.0 to 11.0. Proteolytic enzymes showed extreme stability towards non-ionic surfactants (5 % Tween 80 and 5 % Triton X-100) and oxidizing agents (1 % sodium perborate), and relative stability towards anionic surfactant (1 % Sodium dodecyl sulfate (SDS)). They also showed high stability and compatibility with various laundry liquid detergents from Tunisian market. Furthermore, the crude enzyme was stable towards several organic solvents and retained more than 50 % of its original activity after 30 days of incubation at 30 °C in the presence of 50 % (v/v) dimethylsulfoxide (DMSO). Further, proteases from zebra blenny viscera were found to be effective in the deproteinization of shrimp wastes. The protein removal after 3 h at 40 °C with an enzyme/substrate ratio (E/S) of 5 U/mg protein was about 77 %.







Similar content being viewed by others
References
Anwar A, Saleemuddin M (1998) Alkaline proteases: a review. Bioresour Technol 64:175–183
Banerjee UC, Sani RK, Azmi W, Soni R (1999) Thermostable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Process Biochem 35:213–219
Banik RM, Prakash M (2004) Laundry detergent compatibility of the alkaline protease from Bacillus cereus. Microbiol Res 159:135–140
Ben Khaled H, Ktari N, Ghorbel-Bellaaj O, Jridi M, Lassoued I, Nasri M (2011) Composition, functional properties and in vitro antioxidant activity of protein hydrolysates prepared from sardinelle (Sardinella aurita) muscle. J Food Sci Technol. doi:10.1007/s13197-011-0544-4
Bezerra RS, Lins EJF, Alencar RB, Paiva PMG, Chaves MEC, Coelho LCBB, JrLB C (2005) Alkaline proteinase from intestine of Nile tilapia (Oreochromis niloticus). Process Biochem 40:1829–1834
Bhaskar N, Suresh PV, Sakhare PZ, Sachindra NM (2007) Shrimp biowaste fermentation with Pediococcus acidolactici CFR2182: optimization of fermentation conditions by response surface methodology and effect of optimized conditions on deproteination/demineralization and carotenoid recovery. Enzyme Microb Technol 40:1427–1434
Bougatef A, Haddar A, Ben Khaled H, Barkia A, Nasri M (2008) Digestive proteinases from Sardinelle (S.aurita): Characteristics, application in preparation of antioxidant protein hydrolysates and evaluation in liquid laundry commercial detergents. In Nasri M (ed) Recent Research Developments in Food by products Technology and biotechnology a special book in the area of Food Chemistry, (Research signpost. ISBN: 978-81-308-0259-6.), PP: 98–117
Cowan D (1996) Industrial enzyme technology. Trends Biotechnol 4:177–178
El-Hadj Ali N, Hmidet N, Ghorbel-Bellaaj O, Fakhfakh-Zouari N, Bougatef A, Nasri M (2011) Solvent-stable digestive alkaline proteinases from Striped Seabream (Lithognathus mormyrus) viscera: characteristics, application in the deproteinization of shrimp waste, and evaluation in laundry commercial detergents. Appl Biochem Biotechnol 164:1096–1110
Espósito TS, Amaral IPG, Buarque DS, Oliveira GB, Carvalho LB Jr, Bezerra RS (2009a) Fish processing waste as a source of alkaline proteases for laundry detergent. Food Chem 112:125–130
Espósito TS, Amaral IPG, Marcuschi M, Carvalho LB Jr, Bezerra RS (2009b) Surfactants-and oxidants-resistant alkaline proteases from common carp (cyprinus carpio L.) processing waste. J Food Biochem 33:821–834
Garcia-Carreno FL, Dimes LE, Haard NF (1993) Substrate-gel electrophoresis for composition and molecular weight of proteinases or proteinaceous proteinase inhibitors. Anal Biochem 214:65–69
Ghorbel-Bellaaj O, Hmidet N, Jellouli K, Younes I, Maâlej H, Hachicha R, Nasri M (2011a) Shrimp waste fermentation with Pseudomonas aeruginosa A2: optimization of chitin extraction conditions through Plackett–Burman and response surface methodology approaches. Int J Biol Macromol 48:596–602
Ghorbel-Bellaaj O, Jellouli K, Younes I, Manni L, Ouled Salem M, Nasri M (2011b) A solvent-stable metalloprotease produced by Pseudomonas aeruginosa A2 Grown on shrimp shell waste and its application in chitin extraction. Appl Biochem Biotechnol 164:410–425
Ghosh S (2008) Interaction of trypsin with sodium dodecyl sulfate in aqueous medium: a conformational view. Colloids Surf 66:178–186
Gildberg A (1992) Recovery of proteinases and protein hydrolysates from fish viscera. Bioresour Technol 39:271–276
Gupta MN (1992) Enzyme function in organic solvents. Eur J Biochem 203:25–32
Gupta R, Gupta K, Saxena RK, Khan S (1999) Bleach-stable alkaline protease from Bacillus sp. Biotechnol Lett 21:135–138
Gupta R, Beg Q, Lorenz P (2002) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl Microbiol Biotechnol 59:15–32
Heymer A (1985) Morphologie, coloration, intersexualité et anatomie des organes reproducteurs chez Blennius basiliscus (Teleostei, Blenniidae). Revue Française d’Aquariologie 12:39–52
Jellouli K, Bougatef A, Daassi D, Balti R, Barkia A, Nasri M (2009) New alkaline trypsin from the intestine of grey triggerfish (Balistes capriscus) with high activity at low temperature: purification and characterisation. Food Chem 116:644–650
Jo GH, Jung WJ, Kuk JH, Oh KT, Kim YJ, Park RD (2008) Screening of protease-producing Serratia marcescens FS-3 and its application to deproteinization of crab shell waste for chitin extraction. Carbohydr Polym 74:504–508
Kembhavi AA, Kulkarni A, Pant A (1993) Salt-tolerant and thermostable alkaline protease from Bacillus subtilis NCIM No.64. Appl Biochem Biotechnol 38:83–92
Klomklao S, Benjakul S, Visessanguan W (2004) Comparative studies on proteolytic activity of splenic extracts from three tuna species commonly used in Thailand. J Food Biochem 28:355–372
Ktari N, Fakhfakh N, Balti R, Ben Khaled H, Nasri M, Bougatef A (2012) Effect of degree of hydrolysis and protease type on the antioxidant activity of protein hydrolysates from cuttlefish (Sepia officinalis) by-products. J Aquat Food Prod Technol. doi:10.1080/1049850.2012.658961
Kumar CG, Takagi H (1999) Microbial alkaline proteases from a bioindustrial viewpoint. Biotech Adv 17:561–594
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Male R, Lorens LB, Smalas AO, Torrissen KR (1995) Molecular cloning and characterization of anionic and cationic variants of trypsin from Atlantic salmon. Eur J Biochem 232:677–685
Manni L, Jellouli K, Ghorbel-Bellaaj O, Agrebi R, Haddar A, Sellami-Kamoun A, Nasri M (2010) An oxidant- and solvent-stable protease produced by Bacillus cereus SV1: application in the deproteinization of shrimp wastes and as a laundry detergent additive. Appl Biochem Biotechnol 160:2308–2321
Maurer KH (2004) Detergent peptidases. Curr Opin Biotechnol 15:330–334
Moreira KA, Albuquerque BF, Teixeira MFS, Porto ALF, Lima Filho JL (2002) Application of protease from Nocardiopsis sp. as a laundry detergent additive. World J Microbiol Biotechnol 18:307–312
Rao MS, Muñoz J, Stevens WF (2000) Critical factors in chitin production by fermentation of shrimp biowaste. Appl Microbiol Biotechnol 54:808–813
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632
Roberts GAF (1992) Chitin Chemistry. Macmillan, London
Samal BB, Kara B, Stabinsky Y (1990) Stability of two novel serine proteinases in commercial laundry detergent formulations. Biotechnol Bioeng 35:650–652
Shahidi F, Kamil YVAJ (2001) Enzymes from fish and aquatic invertebrates and their application in the food industry. Trends Food Sci Technol 12:435–464
Shahidi F, Synowiecki J (1991) Isolation and characterization of nutrients and value-added products from snow crab (Chinoecetes opilio) and shrimp (Pandalus borealis) processing discards. J Agric Food Chem 39:1527–1532
Sila A, Nasri R, Bougatef A, Nasri M (2011) Digestive alkaline proteases from the goby (Zosterisessor ophiocephalus) characterization and potential application as detergent additive and in the deproteinization of shrimp wastes. J Aquat Food Prod Technol 21:118–133
Simpson BK (2000) Digestive proteases from marine animals. In: Haard NF, Simpson BK (eds) Seafood enzymes. Marcel Dekker, New York, pp 191–213
Sini TK, Santhosh S, Mathew PT (2007) Study on the production of chitin and chitosan from shrimp shell by using Bacillus subtilis fermentation. Carbohydr Res 342:2423–2429
Acknowledgments
This work was funded by the Ministry of Higher Education and Scientific Research, Tunisia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ktari, N., Khaled, H.B., Younes, I. et al. Zebra blenny (Salaria basilisca) viscera as a source of solvent-stable proteases: characteristics, potential application in the deproteinization of shrimp wastes and evaluation in liquid laundry commercial detergents. J Food Sci Technol 51, 3094–3103 (2014). https://doi.org/10.1007/s13197-012-0817-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-012-0817-6