Abstract
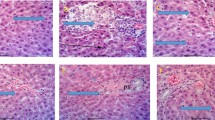
The aim of the current investigation was to evaluate the effects of administration various levels (400, 800 and 1,200 ppm) of pomposia extracts as natural antioxidant in comparison with BHT as synthetic antioxidant on some biochemical activities and histopathological examination of rats. Some of biochemical tests i.e. Alkaline phosphatase, transaminases]Aspartate transferase (AST) and alanine transferase (ALT) [,bilirubin, urea and uric acid were conducted. Histopathological examinations were carried out on the liver and kidney tissue of rats administrated tested substances. The biochemical results indicated that the administration of polyphenolic compounds present in pomposia juice did not cause any significant (p ≥ 0.05) changes in the biochemical parameters whereas the administration of BHT at 200 ppm caused significant (p ≤ 0.05) increase in the activities of enzymes relevant to the functions of liver and kidney. Microscopically examinations of liver and kidney of rat administered various levels of pomposia juice had the same character as that of control rats (this means that the polyphenolic compounds present in pomposia juice did not cause any adverse affect in liver and kidney), in contrast the administration of 200 ppm of BHT caused marked pathological changes in liver and kidney of rats. The results of the current investigation suggest using pomposia juice as safe food grade substance.

Similar content being viewed by others
References
Abd El Mageid MM, Salama NA, Saleh MAM, Abo Taleb HM (2009) Antioxidant and antimicrobial characteristics of red and brown algae extracts 4th Conference on Recent Technologies in Agriculture. Cairo Univerisity, Faculty of Agriculture, pp 818–828
Achrekar S, Kaklij GS, Pote MS, Kelkar SM (1991) Hypoglycemic activity of Eugenia jambolana and Ficus bengalensis: mechanism of action. In Vivo 5:133–148
Ahmad I (1999) Chemistry and technology of deep-frying oils Pak. J Food Sci 9:24–29
Ali RFM (2004) Biochemical Studies on some heated vegetable oils. Master of Science thesis, Biochemistry dept., Faculty of Agriculture, Cairo University, Egypt
Ali RFM (2010) Effect of Pomposia (Syzygium cumini) fruit juice on the stability of fried sunflower oil. J Food Technol 8(2):30–38
AOAC (2000) Official methods of analysis of the Association of Official Analytical Chemists, 17th edn.In Horwitz W (ed) Washington, DC
Barham D, Trinder P (1972) Enzymatic, colorimatric method for determination uric acid in serum plasma and urine. Analyst 97:142–146
Baron DN (1987) A short textbook of chemical pathology, 4th edition- English Language Book Society/Hodder and Stoughton Ltd, Mill Road, Dunton Green, Sevenoaks, Kent, Great Britain, pp 188–228
Belfield A, Goldberg DM (1971) A colorimetric method for determination of alkaline phosphatase in serum. Enzyme 12:561–565
Bergmeyer HU, Harder M (1986) A colorimetric method of determination of serum glutamic oxaloacetic and pyruvic tranaminase. Clin Biochem 24:28–34
Blum M (1996) Designing food for better health. Int Food Ingredients 3:25–29
Chan KM, Decker EA, Means WJ (1993) Extraction and activity of carnosine, a naturally occurring antioxidant in beef muscle. J Food Sci 58:1–4
Chang SS, Ostric-Matijasevic B, Hsieh OAL, Cheng LH (1977) Natural antioxidants from rosemary and sage. J Food Sci 42(4):1102–1106
Chowdhury P, Ray RC (2007) Fermentation of Jamun (Syzgium cumini L.) fruits to form red wine. Asean Food J 14(1):15–23
Culling CFA (1965) Hand book of histopathological techniques, 2nd edn. Butterworth, London
Cuvelier ME, Berset C, Richard H (1994) Antioxidant constituents in sage (Salvia officinalis). J Agric Food Chem 42:655–669
Farag RS, Mohamoud EA, Basuny AM, Ali RFM (2006) Influence of crude olive leaf juice on rat liver and kidney functions. Int J Food Sci Tech 41:1–10
Farag RS, Mohamoud EA, Basuny AM (2007) Use crude olive leaf juice as a natural antioxidant for the stability of sunflower oil during heating. Int J Food Technol 42:107–111
Fawcett JK, Scott JE (1960) Enzymatic, colorimetric method for determination urea in serum, plasma and urine. J Clin Pathol 13:156–162
Ghiselli A, Nardini M, Baldi A, Scaccini C (1998) Antioxidant activity of different phenolic fractions separated from an Italian red wine. J Agric Food Chem 46:361–367
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley, New York
Hamed HS Mattuke, Hemmat I, Attia EA (2001) Production of anthocyanins from pompozia fruitsEgyptian. J Agric Res 79(1):233–243
Hewawasam RP, Jayatilaka KAPW, Pathirana C, Mudduwa LKB (2004) Hepatoprotective effect of Epaltes divaricata extract on carbon tetrachloride induced hepatotoxicity in mice. Ind J Med Res 120:30–34
Innawong B, Mallikarjunan P, Irundayaraj J, Marcy JE (2004) The determination of frying oil quality using Fourier transform infrared attenuated total reflectance. Lebensm Wiss U Technol 37:23–28
Jayaprakasha G, Selvi T, Sakariah K (2003) Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extracts. Food Res Int 36:117–122
Kamei H, Hashimoto Y, Koide T, Kojima T, Hasegawa M (1998) Anti-tumor effect of methanol extracts from red and white wines. Cancer Biother Radiopharm 13:447–452
Kanner J, Harel S, Jaffe R (1991) Lipid peroxidation of muscle food as affected by NaCl. J Agric Food Chem 39:1017–1021
Kong J-M, Chia L-S, Goh N-K, Chia TF, Brouillard R (2003) Analysis and biological activities of anthocyanins. Phytochemistry 64:923–933
Lago ES, Gomes E, da Silva R (2004) Extraction and anthocyanic pigment quantification of the Jamun fruit (Syzygium cumini Lamark). http://www.iqsc.usp.br/outros/eventos/2004/bmcfb/trabalhos/docs/trab-76.pdf.
Lee YB, Kim YS, Ashmore CR (1986) Antioxidant property in ginger rhizome and its application to meat products. J Food Sci 51:20–23
Lin FS, Warner CR, Fazio T (1981) Alteration of phenolic antioxidants in heated vegetable oil. J Am Chem Soc 65:789–792
Lin HM, Yen FL, Ng LT, Lin CC (2007) Protective effects of Ligustrum lucidum fruit extract on acute butylated hydroxytoluene-induced oxidative stress in rats. J Ethnopharmacol 111:129–136
Liya L, Lynn SA, Shiuan C, Caroline K, Aftab A, Navindra PS (2009) Eugenia jambolana Lam. Berry extract inhibits growth and induces apoptosis of human breast cancer but not non-tumorigenic breast cells. J Agric Food Chem 11; 57(3):826–831
Morton J (1987) Fruits of warm climates. Miami, FL, 375
Nadkarni AK (1954) Indian Materia Medica, vol. 1. Popular Prakashan, Bombay, p 1331
Obi FO, Usenu IA, Osayande JO (1998) Prevention of CCl4-induced hepatotoxicity in the rat by H. rosasinensis anthocyanin extracts administered in ethanol. Toxicology 131:93–98
Plaa GL, Hewitt WR (1982) Detection and evaluation of chemical induced liver injury. In: Hayes AW (ed) Principles and methods of toxicology. Raven, New York, p 407
Rajesh MG, Latha MS (2004) Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. J Ethnopharmacol 91:99–104
Ravi K, Ramachandran B, Subramanian S (2004) Effect of Eugenia Jambolana seed kernel on antioxidant defense system in streptozotocin-induced diabetes in rats. Life Sci 75:2717–2731
Rhee KS, Anderson LM, Sams AR (1996) Lipid oxidation potential of beef, chicken and pork. J Food Sci 61:8–12
Sato K, Hegarty GR (1971) Warmed-over flavour in cooked meat. J Food Sci 36:1098–1102
Scartezzini P, Speroni E (2000) Review on some plants of Indian traditional medicine with antioxidant activity. J Ethnopharmacol 71(1–2):23–43
Shahjahan MK, Sabitha JM, Shyamala-Devi CS (2004) Effect of Solanum trilobatum against carbon tetrachloride induced hepatic damage in albino rats. Indian J Med Res 120:194–198
Sharma JN, Sheshadri TR (1955) Survey of anthocyanins from Indian sources: Part II. J Sci Ind Res 14:211–214
Sharma B, Viswanath G, Salunke R, Roy P (2008) Effects of flavonoid-rich extract from seeds of Eugenia jambolana (L.) on carbohydrate and lipid metabolism in diabetic mice. Food Chem 110:697–705
Teixeira CC, Blotta RM, Costa AP, Mussnich DG, Ranquetat GG, Fuchs FD (1992) Plants employed in the treatment of diabetes mellitus results of an ethnopharmacological survey in Porto Alegre. Brazil Fitoterapia LXIII 4:320–322
Timberlake CF, Bridle P (1982) Distribution of anthocyanins in food plant. In: Markakis P (ed) Anthocyanins as food colors. Academic, New York, p 137
Veigas JM, Narayan MS, Laxman PM, Neelwarne B (2007) Chemical nature, stability and bioefficacies of anthocyanins from fruit peel of Syzygium cumini skeels. Food Chem 105:619–627
Venkateswarlu G (1952) On the nature of the colouring matter of the jambul fruit (Eugenia jambolana). J Indian Chem Soc 29(6):434–437
Vizzotto M, Pereira MC (2008) Characterization of functional properties of Jambolan (Caracterização das Propriedades Funcionais do Jambolão). Bol Pesqui Desenvolvimento 79:1–27
Walter M, Gerade H (1970) A colorimetric method for determination bilirubin in serum and plasma. Micro Chem J 15:231–236
Wright PJ, Plummer DT (1974) The use of urinary enzyme measurement to detect renal damage caused by nephrotoxic compounds. Biochem Pharmacol 23(1):65–73
Yanardag R, Tunali S (2006) Vanadyl sulfate administration protects the streptozotocin-induced oxidative damage to brain tissue in rats. Mol Cell Biochem 286:153–159
Yin MC, Cheng WS (1997) Oxymyoglobin and lipid oxidation in phosphatidylcholine liposomes retarded by α- tocopherol and b-carotene. J Food Sci 62:1095–1097
Yoshinaga M, Yamakawa O, Nakatani M (1999) Genotypic diversity of anthocyanin content and composition in purple-fleshed sweet potato (Ipomoea batatas (L.) Lam). Breed Sci 49:43–47
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Anany, A.M., Ali, R.F.M. Biochemical and histopathological effects of administration various levels of Pomposia (Syzygium cumini) fruit juice as natural antioxidant on rat health. J Food Sci Technol 50, 487–495 (2013). https://doi.org/10.1007/s13197-011-0372-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-011-0372-6