Abstract
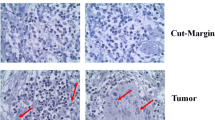
The locoregional recurrence in oral cancer is not predicted by the histopathological parameters solely as the normal morphological looking cells harbor the genomic instability which acts as the potential tumor cells for recurrence in future. Therefore, there is an urgent need of the biomarker for prognostic stratification of patients with high risk of disease recurrence and appropriate management. Eighty oral squamous cell carcinoma (OSCC) patients were included in the study during the period 2012 to 2014 at Apollo Hospitals and Kalinga Institute of Medical sciences, Bhubaneswar. OSCC tissue samples were collected at the time of surgical excision, and immunohistochemistry (IHC) was performed to check the expression of β-catenin in cut margin (CM) and tumor. Statistical analysis was carried out using SPSS based on clinical and pathological records. It was observed that among 80 patients, 33.75% (27 patients) developed recurrence. The recurrence rate was low for 6 out of 27 patients (22.2%) where β-catenin is positive in tumor and negative in cut margin, while it was quite high in 21 out of 27 (77.8%) when marker is negative in tumor but positive in cut margin (CM). The odds of recurrence among patients having high levels of 𝛽-catenin in CM was 3.6 times higher than the odds of recurrence among patients having lower levels of 𝛽-catenin in CM (p < 0.017). In conclusion, this study highlighted that 𝛽-catenin can be included as a prognostic molecular marker, along with routine histopathological study to influence therapeutic decisions and appropriate management of disease.


Similar content being viewed by others
References
Pathare SM et al (2011) Clinicopathological and prognostic implications of genetic alterations in oral cancers. Oncol Lett 2(3):445–451
Sankaranarayanan R (1990) Oral cancer in India: an epidemiologic and clinical review. Oral Surg Oral Med Oral Pathol 69(3):325–330
Pakhale SS, Maru GB (1998) Distribution of major and minor alkaloids in tobacco, mainstream and sidestream smoke of popular Indian smoking products. Food Chem Toxicol 36(12):1131–1138
Viswanatha C, Hedne SN, Hasan S (2018) Correlation between histological grading, LVI and PNI of carcinoma oral tongue to lymph node metastasis. 5(1):6
Adel M, Kao HK, Hsu CL, Huang JJ, Lee LY, Huang Y, Browne T, Tsang NM, Chang YL, Chang KP (2015) Evaluation of lymphatic and vascular invasion in relation to clinicopathological factors and treatment outcome in oral cavity squamous cell carcinoma. Medicine 94(43):e1510
Lin Y-T, Chien CY, Lu CT, Lou SD, Lu H, Huang CC, Fang FM, Li SH, Huang TL, Chuang HC (2015) Triple-positive pathologic findings in oral cavity cancer are related to a dismal prognosis. Laryngoscope 125(9):E300–E305
Jardim JF, Francisco ALN, Gondak R, Damascena A, Kowalski LP (2015) Prognostic impact of perineural invasion and lymphovascular invasion in advanced stage oral squamous cell carcinoma. Int J Oral Maxillofac Surg 44(1):23–28
Park BJ, Chiosea SI, Grandis JR (2010) Molecular changes in the multistage pathogenesis of head and neck cancer. Cancer Biomark 9(1–6):325–339. https://doi.org/10.3233/CBM-2011-0163
Leemans CR, Braakhuis BJ, Brakenhoff RH (2011) The molecular biology of head and neck cancer. Nat Rev Cancer 11(1):9–22
Wise-Draper TM, Draper DJ, Gutkind JS, Molinolo AA, Wikenheiser-Brokamp KA, Wells SI (2012) Future directions and treatment strategies for head and neck squamous cell carcinomas. Transl Res 160(3):167–177
Strickaert A, Saiselet M, Dom G, de Deken X, Dumont JE, Feron O, Sonveaux P, Maenhaut C (2017) Cancer heterogeneity is not compatible with one unique cancer cell metabolic map. Oncogene 36(19):2637–2642
Padhi S, Saha A, Kar M, Ghosh C, Adhya A, Baisakh M, Mohapatra N, Venkatesan S, Hande MP, Banerjee B (2015) Clinico-pathological correlation of β-catenin and telomere dysfunction in head and neck squamous cell carcinoma patients. J Cancer 6(2):192–202
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Hoffmeyer K, Raggioli A, Rudloff S, Anton R, Hierholzer A, del Valle I, Hein K, Vogt R, Kemler R (2012) Wnt/ -catenin signaling regulates telomerase in stem cells and cancer cells. Science 336(6088):1549–1554
Iwai S, Yonekawa A, Harada C, Hamada M, Katagiri W, Nakazawa M, Yura Y (2010) Involvement of the Wnt-β-catenin pathway in invasion and migration of oral squamous carcinoma cells. Int J Oncol 37(5):1095–1103
Park J-I, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, Chang W, Meng Z, Cheung P, Ji H, McLaughlin M, Veenstra TD, Nusse R, McCrea PD, Artandi SE (2009) Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460(7251):66–72
Padhi SS, Roy S, Kar M, Saha A, Roy S, Adhya A, Baisakh M, Banerjee B (2017) Role of CDKN2A/p16 expression in the prognostication of oral squamous cell carcinoma. Oral Oncol 73:27–35
Roy S, Kar M, Roy S, Saha A, Padhi S, Banerjee B (2018) Role of beta-catenin in cisplatin resistance, relapse and prognosis of head and neck squamous cell carcinoma. Cell Oncol (Dordr) 41(2):185–200
Shiah SG, Shieh YS, Chang JY (2016) The role of Wnt signaling in squamous cell carcinoma. J Dent Res 95:129–134
Rampias T, Pectasides E, Prasad M, Sasaki C, Gouveris P, Dimou A, Kountourakis P, Perisanidis C, Burtness B, Zaramboukas T, Rimm D, Fountzilas G, Psyrri A (2013) Molecular profile of head and neck squamous cell carcinomas bearing p16 high phenotype. Ann Oncol 24(8):2124–2131
Hameid MA (2016) β-Catenin expression in perilesional area of different grades of oral squamous cell carcinoma. Sulaimani Dent J 3(1):25–29
Cai ZG, Shi XJ, Gao Y, Wei MJ, Wang CY (2008) Yu GY: β-catenin expression pattern in primary oral squamous cell carcinoma. Chin Med J 121:1866–1870
Padhi S, Saha A, Kar M et al (2015) Clinico-pathological correlation of β-catenin and telomere dysfunction in head and neck squamous cell carcinoma patients. J Cancer 6(2):192–202. Published 2015 Jan 15. https://doi.org/10.7150/jca.9558
Padhi SS, Kar M, Saha A, Baisakh MR, Mohapatra N, Banerjee BN (2014) Rapid recurrence and poor prognosis with altered levels of β-catenin in 3 cases of head and neck squamous cell carcinoma patients. Head Neck Oncol 6:39
Mitra A, Mishra L, Li S (2015) EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 6:10697–10711
Westra WH, Sidransky D (1998 Oct 21) Phenotypic and genotypic disparity in premalignant lesions: of calm water and crocodiles. J Natl Cancer Inst 90(20):1500–1501
Clark DJ, Li M (2017) Understanding the surgical margin - a molecular assessment. Oral Maxillofacial Surg Clin N Am 29:245–258
Caldwell RL, Gonzalez A et al (2006) Molecular assessment of the tumor protein microenvironment using imaging mass spectrometry. CANCER GENOMICS PROTEOMICS:279–287
Balog J, Szaniszlo T, Schaefer KC, Denes J, Lopata A, Godorhazy L, Szalay D, Balogh L, Sasi-Szabo L, Toth M, Takats Z (2010 Sep 1) Identification of biological tissues by rapid evaporative ionization mass spectrometry. Anal Chem 82(17):7343–7350. https://doi.org/10.1021/ac101283x
Balog J et al (2013) Intraoperative tissue identification using rapid evaporative ionization mass spectrometry. Sci Transl Med 5(194):194ra93
Acknowledgments
The authors acknowledge the Department of Biotechnology, Government of India for providing grant and technical support of Molecular Stress and Stem Cell Biology (MSSB) group to carry out this study.
Funding
This study was supported by grant from Department of Biotechnology, Government of India, Grant No- BT/PR 17576/MED/30/1690/2016, [Virtual National Oral Cancer Institute (Understanding the Disease Biology and Epigenetic Diversity of Oral Cancer in India: Implications for New Diagnostics and Therapeutics)].
Author information
Authors and Affiliations
Contributions
MK carried out the surgery, planned the experiments, interpreted the clinical data, and wrote the manuscript. MS performed the data analysis, interpreted the data, and wrote the manuscript. SR and SP collected tissues, processed for experimental analysis, accumulated the data, and helped in organizing the manuscript. BNB conceived the idea, guided through the experiments, and wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Table 1
Clinicopathological details of OSCC patients (n = 80). (DOCX 16 kb)
Supplementary Table 2
TNM staging of 80 OSCC patients. (DOCX 11 kb)
Rights and permissions
About this article
Cite this article
Kar, M., Sultania, M., Roy, S. et al. 𝛽-Catenin—a Possible Prognostic Molecular Marker for Recurrence in Histopathologically Negative Surgical Margin of Oral Cancer. Indian J Surg Oncol 12 (Suppl 1), 128–133 (2021). https://doi.org/10.1007/s13193-020-01217-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-020-01217-0