Abstract
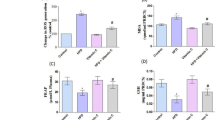
Vitamin A plays physiological and antioxidants properties and is associated with protective effects on arterial level. However, deleterious effects have been reported, including those observed by our group, which has demonstrated pro-oxidant properties in other systems. Therefore, it is needed to better understand the redox effects of retinoids on arterial system. Thus, our aim was to compare vascular redox parameters among animals supplemented or not with vitamin A. Eighty-five adult male rats were treated with different retinyl palmitate doses (1,000–9,000 IU kg−1 day−1) or saline for 3 (25 rats, n = 5 for each group), 7 (25 rats, n = 5 for each group), and 28 (35 rats, n = 7 for each group) days periods. Aorta artery was surgically removed, cleaned to remove the blood, and homogenized. It was evaluated thiobarbituric reactive species (TBARS), total reduced sulfhydryl (SH), and activities of superoxide dismutase (SOD) and catalase (CAT). Statistics were conducted by one-way ANOVA with Dunnet’s post hoc and significance value of p ≤ 0.05. About TBARS, we observed no modifications after 3 days, but a decrease after 7 days in all doses and after 28 days in three higher doses. The two higher doses yielded an increase on SH only after 3 days. SOD activity decreased in three higher doses after 3 days and in all doses after 28 days, but no modifications after 7 days, while CAT activity increased in all doses after 3 days, decreased in all doses after 7 days, and did not change after 28 days. In conclusion, vitamin A induces antioxidant status on vascular level.




Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Bannister JV, Calabrese L (1987) Assays for superoxide dismutase. Methods Biochem Anal 32:279–312
da Rocha RF, de Oliveira MR, Schonhofen P, Schnorr CE, Dal Pizzol F, Moreira JC (2010) Long-term vitamin A supplementation at therapeutic doses induces mitochondrial electrons transfer chain (METC) impairment and increased mitochondrial membrane-enriched fraction (MMEF) 3-nitrotyrosine on rat heart. Free Radic Res 44:505–512
Dal-Pizzol F, Klamt F, Frota ML Jr, Moraes LF, Moreira JC, Benfato MS (2000) Retinol supplementation induces DNA damage and modulates iron turnover in rat Sertoli cells. Free Radic Res 33:677–687
Dal-Pizzol F, Klamt F, Benfato MS, Bernard EA, Moreira JC (2001) Retinol supplementation induces oxidative stress and modulates antioxidant enzyme activities in rat sertoli cells. Free Radic Res 34:395–404
Dal-Pizzol F, Klamt F, Dalmolin RJ, Bernard EA, Moreira JC (2001) Mitogenic signaling mediated by oxidants in retinol treated Sertoli cells. Free Radic Res 35:749–755
De Oliveira MR, Moreira JC (2008) Impaired redox state and respiratory chain enzyme activities in the cerebellum of vitamin A-treated rats. Toxicology 253:125–130
de Oliveira MR, Silvestrin RB, Mello EST, Moreira JC (2007) Oxidative stress in the hippocampus, anxiety-like behavior and decreased locomotory and exploratory activity of adult rats: effects of sub acute vitamin A supplementation at therapeutic doses. Neurotoxicology 28:1191–1199
de Oliveira MR, Silvestrin RB, Mello e Souza T, Moreira JC (2008) Therapeutic vitamin A doses increase the levels of markers of oxidative insult in substantia nigra and decrease locomotory and exploratory activity in rats after acute and chronic supplementation. Neurochem Res 33:378–383
De Oliveira MR, Oliveira MW, Da Rocha RF, Moreira JC (2009) Vitamin A supplementation at pharmacological doses induces nitrosative stress on the hypothalamus of adult Wistar rats. Chem Biol Interact 180:407–413
de Oliveira MR, Oliveira MW, Lorenzi R, Fagundes da Rocha R, Fonseca Moreira JC (2009) Short-term vitamin A supplementation at therapeutic doses induces a pro-oxidative state in the hepatic environment and facilitates calcium-ion-induced oxidative stress in rat liver mitochondria independently from permeability transition pore formation: detrimental effects of vitamin A supplementation on rat liver redox and bioenergetic states homeostasis. Cell Biol Toxicol 25:545–560
de Oliveira MR, Soares Oliveira MW, Muller Hoff ML, Behr GA, da Rocha RF, Fonseca Moreira JC (2009) Evaluation of redox and bioenergetics states in the liver of vitamin A-treated rats. Eur J Pharmacol 610:99–105
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Frota ML Jr, Klamt F, Dal-Pizzol F, Schiengold M, Moreira JC (2004) Retinol-induced mdr1 and mdr3 modulation in cultured rat Sertoli cells is attenuated by free radical scavengers. Redox Rep 9:161–165
Gatica L, Alvarez S, Gomez N, Zago MP, Oteiza P, Oliveros L, Gimenez MS (2005) Vitamin A deficiency induces prooxidant environment and inflammation in rat aorta. Free Radic Res 39:621–628
Gatica LV, Vega VA, Zirulnik F, Oliveros LB, Gimenez MS (2006) Alterations in the lipid metabolism of rat aorta: effects of vitamin a deficiency. J Vasc Res 43:602–610
Gelain DP, de Bittencourt Pasquali MA, Zanotto-Filho A, de Souza LF, de Oliveira RB, Klamt F, Moreira JC (2008) Retinol increases catalase activity and protein content by a reactive species-dependent mechanism in Sertoli cells. Chem Biol Interact 174:38–43
Gutteridge JMC, Halliwell B (2001) Free radicals in biology and medicine. Oxford University Press Inc., New York
Haxsen V, Adam-Stitah S, Ritz E, Wagner J (2001) Retinoids inhibit the actions of angiotensin II on vascular smooth muscle cells. Circ Res 88:637–644
Klamt F, Dal-Pizzol F, Bernard EA, Moreira JC (2003) Enhanced UV-mediated free radical generation; DNA and mitochondrial damage caused by retinol supplementation. Photochem Photobiol Sci 2:856–860
Lam HS, Chow CM, Poon WT, Lai CK, Chan KC, Yeung WL, Hui J, Chan AY, Ng PC (2006) Risk of vitamin A toxicity from candy-like chewable vitamin supplements for children. Pediatrics 118:820–824
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lu L, Yao T, Zhu YZ, Huang GY, Cao YX, Zhu YC (2003) Chronic all-trans retinoic acid treatment prevents medial thickening of intramyocardial and intrarenal arteries in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 285:H1370–H1377
Meyer AJ, Hell R (2005) Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynth Res 86:435–457
Montrone M, Martorelli D, Rosato A, Dolcetti R (2009) Retinoids as critical modulators of immune functions: new therapeutic perspectives for old compounds. Endocr Metab Immune Disord Drug Targets 9:113–131
Myhre AM, Carlsen MH, Bohn SK, Wold HL, Laake P, Blomhoff R (2003) Water-miscible, emulsified, and solid forms of retinol supplements are more toxic than oil-based preparations. Am J Clin Nutr 78:1152–1159
Omenn GS, Goodman G, Thornquist M, Grizzle J, Rosenstock L, Barnhart S, Balmes J, Cherniack MG, Cullen MR, Glass A et al (1994) The beta-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high risk populations: smokers and asbestos-exposed workers. Cancer Res 54:2038s–2043s
O’Neil C, Shevill E, Chang AB (2008) Vitamin A supplementation for cystic fibrosis. Cochrane Database Syst Rev: CD006751
Peluffo G, Radi R (2007) Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc Res 75:291–302
Smith MA, Parkinson DR, Cheson BD, Friedman MA (1992) Retinoids in cancer therapy. J Clin Oncol 10:839–864
Sporn MB, Roberts AB, Goodman DS (1994) The retinoids: biology, chemistry and medicine. Raven Press, New York
Stone JR, Yang S (2006) Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 8:243–270
Van Zander J, Orlow SJ (2005) Efficacy and safety of oral retinoids in psoriasis. Expert Opin Drug Saf 4:129–138
Zanotto-Filho A, Schroder R, Moreira JC (2008) Xanthine oxidase-dependent ROS production mediates vitamin A pro-oxidant effects in cultured Sertoli cells. Free Radic Res 42:593–601
Zanotto-Filho A, Gelain DP, Schroder R, Souza LF, Pasquali MA, Klamt F, Moreira JC (2009) The NF kappa B-mediated control of RS and JNK signaling in vitamin A-treated cells: duration of JNK-AP-1 pathway activation may determine cell death or proliferation. Biochem Pharmacol 77:1291–1301
Acknowledgments
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico/National Council for Research and Development (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior/Coordination for Improvement of Superior Level Personnel (CAPES) and from Rede Instituto Brasileiro de Neurociência/Net Brazilian Institute of Neuroscience (IBN-Net)—01.06.0842-00. We would also like to thank the UFRGS, a public university from Brazil, where the study was performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Rocha, R.F., de Oliveira, M.R., Schonhofen, P. et al. Vitamin A supplementation for different periods alters rat vascular redox parameters. J Physiol Biochem 66, 351–357 (2010). https://doi.org/10.1007/s13105-010-0041-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-010-0041-7