Abstract
The estrogen receptor α (ERα) splicing variant with an in-frame deletion of exon 3 (ERΔ3) is frequently expressed in the normal breast, but its influence on tumorigenesis has not been explored. In vitro, ERΔ3 has dominant negative activity, suggesting it may suppress estrogen stimulation in the breast. ERΔ3 may inhibit classical signaling on estrogen response element (ERE)-regulated genes as well as activate non-classical pathways at Sp1 and AP-1 sites. Transgenic mice were developed that express mouse ERΔ3 in all tissues examined, including the mammary gland. To investigate if ERΔ3 expression affects tumorigenesis, ERΔ3 mice were crossbred with MMTV-Neu mice. Mammary tumor onset was significantly delayed in ERΔ3/Neu versus MMTV-Neu females and metastatic incidence and burden was significantly reduced. Consequently, ERΔ3 expression suppressed tumor development and metastasis in this aggressive model of HER2/Neu-positive breast cancer. To determine if ER ligands with anticancer activity may augment ERΔ3 protection, the bitransgenic mice were treated with tamoxifen and soy isoflavones starting at age 2 months. Soy protein with isoflavones (181 mg/1,800 kcal) did not affect tumor development in MMTV-Neu or ERΔ3/Neu mice; however, metastatic progression was not inhibited in soy-treated ERΔ3/Neu mice, as it was in untreated ERΔ3/Neu mice. In contrast, tamoxifen (20 mg/1,800 kcal) significantly enhanced tumor prevention in ERΔ3/Neu versus MMTV-Neu mice (98 % vs. 81 % tumor free). The results in ERΔ3/Neu mice demonstrate that ERΔ3 influences estrogen-dependent mammary carcinogenesis and, thus, may be protective in women expressing ERΔ3 in the breast. However, exposure to different estrogens may augment or block its beneficial effects.
Similar content being viewed by others
Introduction
Alternative splicing variants for estrogen receptor α (ERα), with one or more exons deleted, are common in normal and neoplastic breast tissue [1]. Although RNA is routinely used to discriminate between wild-type (WT) and variant ERα expression, variant proteins have also been detected in human breast tumors, normal and malignant ovarian tissue, and breast cancer cell lines [1–5]. Many studies have investigated whether ER variants in breast tumors influence endocrine resistance, expression of ERα and progesterone receptor (PR), and tumor behavior [1]. Their presence in normal breast tissue has led to speculation that they may influence estrogen activity and, accordingly, cancer development; however, this potential has not been investigated. Identification of ERα variants with modified functions, such as dominant negative or positive activity, supports the possibility that these altered receptors influence estrogen responsiveness of breast tissue. One splicing variant with dominant negative activity occurs from the in-frame deletion of exon 3 from ERα (ERΔ3) [6], which codes for the second zinc finger of the DNA binding domain (DBD). The ERΔ3 variant binds 17β-estradiol with high affinity, localizes to the nucleus, and dimerizes with ERα [7]; however, it is unable to bind to an estrogen response element (ERE) or to transactivate an ERE–reporter construct [6]. Its dominant negative activity was demonstrated in HeLa cells; co-transfecting a 10:1 ratio of ERΔ3:ERα vectors had 80 % inhibition and a 1:1 ratio had 30 % inhibition of wild-type (WT) ERα activity on an ERE–reporter [6]. Furthermore, the lower ERΔ3 expression in tumors compared to normal breast suggests that loss of ERΔ3 expression may influence breast tumorigenesis [8, 9].
With limited information on how ERα variants act in vivo in normal and malignant estrogen target tissues, the ERΔ3 transgenic mouse model was developed to test the ability of this variant to inhibit estrogen responses. To maintain normal species interactions with DNA elements, other cellular proteins, and wild-type receptors, the mice express mouse ERΔ3 variant (exon 4 or third coding exon in mouse Esr1 is equivalent to exon 3 in human ESR1). The amino acid sequence for this exon is 100 % conserved in the human and mouse ERα genes. Due to the in-frame deletion, other ERα functional domains remain intact, such as nuclear localization, AF-1 and AF-2, ligand-dependent dimerization, and ligand binding. Both mouse and human ERα variants lacking the second zinc finger do not bind to an ERE or stimulate transcription of an ERE–reporter [10]. Two lines of ERΔ3 mice (D and F) express the ERΔ3 transgene in all tissues thus far examined, including in the mammary gland, in which a line F female mice expressed ERΔ3 at lower levels than ERα (0.6:1 ratio), unlike the line D mouse (14:1 ratio) [11]. ERΔ3 mice develop normally, both genders are fertile, and the dams lactate without problems.
To determine if ERΔ3 expression in normal mammary tissue influences tumor development, ERΔ3 mice were crossbred with MMTV-Neu transgenic mice. The MMTV-Neu model expresses the unactivated rat Neu (c-ErbB2) transgene and mimics many features of HER2-positive breast cancer, including stochastic, multistep carcinogenesis; tumor pathology; and frequent metastatic progression [12, 13]. Estrogen is required for Neu-induced tumor development since tamoxifen and ovariectomy effectively prevent tumor formation [14–18]; therefore, MMTV-Neu mice provide a good model to test the inhibitory potential of ERΔ3 on estrogen-dependent events in mammary carcinogenesis.
A primary mechanism of dominant negative inhibition is to form inactive heterodimers with the wild-type (WT) receptors [1], such as ERΔ3:ERα and ERΔ3:ERβ heterodimers. The weak dimerization domain in exon 3 is deleted, but the strong, ligand-dependent dimerization domain remains in ERΔ3 [19]. Therefore, for ERΔ3 to dimerize with ERα or ERβ to block their activity, estrogen must be present. In intact mice, endogenous estrogens would initiate dimerization to inhibit the estrogen responses normally induced by ERα and ERβ and ERΔ3 heterodimers may correspondingly repress estrogen-dependent mammary carcinogenesis. Additionally, other ER ligands with reported anticancer effects may enhance the potential preventative actions of ERΔ3 in mammary tissue. Tamoxifen prevents breast cancer in women [20] and in the MMTV-Neu mouse model tested herein [14–17]. Soy isoflavones, mainly genistein and daidzein, are weak phytoestrogens, which also act as antiestrogens in breast cancer cells [21, 22], and inhibit mammary cancer in MMTV-Neu mice [16, 17, 23]. Therefore, both tamoxifen and soy protein isolate containing isoflavones were tested in intact female mice expressing ERΔ3 to determine if either could enhance the potential inhibitory action of this variant on Neu-induced mammary tumor development and metastatic progression.
Materials and Methods
Animal Care
All animal work was approved by the Institutional Animal Care and Use Committee at Wake Forest University Medical Center, Cedars-Sinai Medical Center, and Duquesne University in accordance with NIH guidelines. Dizygous line F ERΔ3 mice [FVB/N-TgN(mERΔ3os)06Eme] [11] were bred with dizygous MMTV-Neu mice expressing the Neu protooncogene [FVB/N-Tg(MMTVneu)202Mul/J] [12] to generate the bitransgenic ERΔ3/Neu mice (hemizygous for both transgenes). The MMTV-Neu mice (Jackson Laboratory, Bar Harbor, ME, USA) were crossbred with wild-type (WT) mice (FVB/N strain; Jackson Laboratory) to generate the hemizygous MMTV-Neu control mice. The breeders and progeny were maintained on a semi-purified isoflavone-free diet to prevent exposure to these phytoestrogens during all developmental stages of the study mice. The control diet is a modification of AIN-93G using corn oil with 20 % protein, 16 % fat, 64 % carbohydrates, and 3,713 kcal/kg (Harlan-Teklad, Madison, WI, USA).
For the tumor study, 242 MMTV-Neu and 208 ERΔ3/Neu female mice were randomized at weaning into the three treatment groups (control, soy, and tamoxifen) and maintained on the control diet. At 2 months of age, the soy and tamoxifen groups were transferred to treatment diets (Harlan Teklad). The tamoxifen diet contained tamoxifen citrate (Sigma-Aldrich, St. Louis, MO, USA) equivalent to 20 mg/1,800 kcal tamoxifen in the control diet. The tamoxifen dose is based on 20 mg/day for breast cancer prevention [24] and an average woman’s diet of 1,800 kcal. Approximately 0.17 mg/day tamoxifen would be consumed for a mouse eating 4 g of diet/day.
The soy diet contained 21.7 % soy protein isolate (Supro 670, BXP-H-0206; Protein Technologies International, St. Louis, MO, USA) with the same lot used throughout the study. The isoflavone concentrations per kilogram of diet are as follows: 619 mg/kg total conjugated and unconjugated isoflavones, 374 mg/kg isoflavones (aglycones), 191 mg/kg genistein, 143 mg/kg daidzein, and 39 mg/kg glycitein. Soy protein isolate provided the protein and other nutrients, which were equalized to the control diet (20 % protein, 16 % fat, 64 % carbohydrates, and 3,714 kcal/kg) to mimic women consuming soy as their only source of protein (181 mg aglycone isoflavones/1,800 kcal; 1.5 mg/day isoflavones or 0.76 mg/day genistein and 0.57 mg/day daidzein for mice eating 4 g of diet/day). The dose tested in this study is higher than typical consumption in Japanese women, which ranges between 18 and 70 mg/day aglycone isoflavones [25]. Additionally, mice may have higher circulating isoflavone concentrations due to less efficient conjugation [26]. To investigate the effects of soy isoflavones in an estrogen-deficient environment, two groups of MMTV-Neu mice were ovariectomized under inhaled isoflurane anesthesia at age 2 months and then fed either the soy or control diet.
The estrous cycle stage at necropsy was assessed using vaginal smears stained with Dif-stain kit (IMEB Inc., San Marcos, CA, USA). Blood from 3-month-old WT (FVB/N) and ERΔ3 mice in estrus were analyzed for serum 17β-estradiol and progesterone concentrations with the Double Antibody Estradiol and Coat-a-Count Progesterone kits (Siemens, Los Angeles, CA, USA).
Tumor Doubling Time, Volume, and Histopathology
Tumor onset was determined by weekly palpations starting at 4 months of age. For tumor growth, weekly caliper measurements were performed on two dimensions. Tumor doubling time for mice with only one mammary tumor was determined using the formula T 1 − T 0 × log(2)/V 1 − V 0, with T for time in days and V for cubic millimeters of volume, after tumors were at least 18 mm3 in size (volume by length × width2 × 0.523). Lungs were fixed in cold 4 % paraformaldehyde and 6-μm paraffin sections were stained with hematoxylin and eosin; sectioning and staining were performed by Mass Histology Service (Worcester, MA, USA). Sections were examined by a board-certified veterinary pathologist (JMC) to assess the incidence of micrometastases and confirm grossly detected metastatic lesions as previously described [27].
PR Immunohistochemistry
Mammary gland sections from ERΔ3 and WT mice in estrus were heat-treated for antigen retrieval; pretreated with 3 % hydrogen peroxide; blocked with unconjugated secondary antibody (anti-mouse IgG); incubated with the primary progesterone receptor antibody, PR10A9 at 1:50 (Beckman Coulter Inc., Brea, CA, USA), overnight at 4 °C; exposed to biotin–streptavidin link and labeling antibodies, Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA, USA); incubated with DAB chromogen (Biogenex, Fremont, CA, USA); counterstained with Mayer’s hematoxylin; dehydrated; and coverslipped. Immunostained cells were quantified by counting labeled cells, using a stereologic grid filter [28] in random regions of the mammary gland in blinded samples at ×400 magnification. At the grid intersections, nuclei were counted as unlabeled (0), weakly labeled (+), moderately labeled (2+), or intensely labeled (3+). One hundred epithelial cells/animal were counted (two animals had 96 or 98 cells). Sections stained with normal mouse serum (no primary antibody) did not result in positive-stained cells (see Online Resource 1).
RNA Levels
Mammary gland RNA was prepared using the Absolutely RNA RT–PCR miniprep kit (Stratagene, La Jolla, CA, USA) and cDNAs generated with reverse transcriptase (RT) using the qScript cDNA Synthesis Kit (Quanta Bioscience, Gaithersburg, MD, USA). cDNAs were analyzed by real-time RT–PCR in the iCycler (Bio-Rad, Hercules, CA, USA) using BR SYBR Green SuperMix for iQ Systems (Quanta Bioscience) with the primer sequences below for 50 cycles at 95 °C for 30 s, 60 °C for 60 s. Primers for progesterone receptor (Pgr) forward: TGGGAGCTGCAAGGTCTTCT and reverse: TGCCAGCCTGACAACACTTT; estrogen receptor alpha (Esr1) forward: GTCCAGCTACAAACCAATGC and reverse: ATCTCTCTGACGCTTGTGCT; ERΔ3 transgene forward: ATTCAAGGGATCCGCATAC and reverse: ACAAGGCAGGGCTATTCTTC; cytokeratin 18 (Krt18) forward: TTGCGAATTCTGTGGACAAT and reverse: TTCCACAGTCAATCCAGAGC; and cyclophilin A (Ppia) forward: TATCTGCACTGCCAAGACTG and reverse: ACAGTCGGAAATGGTGATCT. Primer sequences, which discriminate between Neu transgene and endogenous Neu gene, were previously reported [27]. Gene expression was normalized to Ppia expression from the same RT reaction. Amplified products were confirmed with no RT controls and melt curve analysis. The proper size amplified product for each primer set was confirmed in a subset of samples by agarose gel electrophoresis.
Statistical Analyses
Chi-squared and Fisher’s exact test were used for categorical variables. Mann–Whitney test was used to compare two groups, one-way ANOVA for three groups, and two-way ANOVA for comparing two variables, i.e., genotype and treatment. Survival curves were analyzed with log-rank test and Gehan–Breslow–Wilcoxon test, which places more weight on early events, such as would occur with changes in latency. Analyses were performed using GraphPad Prism 5.0 software (San Diego, CA, USA). A p value <0.05 designated significance.
Results
Progesterone Receptor Immunostaining in Mammary Epithelium and Circulating Hormone Levels in ERΔ3 Mice
In ERΔ3 mice, lines D and F express the transgene in the mammary gland [11]. Due to the reported dominant negative activity of ERΔ3 [6], its ability to suppress the estrogen-responsive PR in the mammary epithelium was examined in line D and F females. In both lines, strong PR immunostaining was significantly reduced compared to the WT mice (Fig. 1a). Examples of specific and non-specific PR immunostaining from WT and line F ERΔ3 mammary tissue are shown in Online Resource 1. Although not significantly different than WT mice, the number of epithelial cells with no and weak staining was increased in both lines, also suggesting reduced expression of PR. These results suggest ERΔ3 may repress estrogen action in the mammary gland and, therefore, may inhibit cancer development. Due to the stunted growth of dizygous line D mice, which affects breeding with normal size mates and litter sizes, line F females were selected for studying ERΔ3 effects on mammary cancer development. 17β-Estradiol (E2) and progesterone (P4) serum levels in line F females in estrus (Fig. 1b, c) confirmed that E2, but not P4, serum levels were significantly elevated compared to WT mice (p = 0.009, Mann–Whitney), as previously observed in ERΔ3 mice with lines D and F combined [11]. These data indicate that reduced PR immunostaining in the ERΔ3 mammary epithelium occurred even in the presence of higher E2 levels.
Intensity of progesterone receptor immunostaining in mammary epithelium is decreased despite higher 17β-estradiol serum levels in ERΔ3 mice. a Progesterone receptor immunostaining intensity in mammary epithelial cells are shown for wild-type (WT) FVB/N (n = 6) and lines D (n = 6) and F (n = 8) ERΔ3 female mice. Two-way ANOVA showed no significance for genotype, but significance was observed for the level of staining and the interaction of staining and genotype (p < 0.01). Bonferroni tests identified significance between the groups as shown in the graph: a relative to cells without staining (none); b relative to weakly staining cells (weak); c relative to moderately staining cells (mid); and asterisk designates significance compared to the strongly staining cells (strong) in WT mice, p < 0.05. p <0.001 for WT and line F for none vs. strong as well as weak vs. strong and mid vs. strong for WT; p <0.01 for weak vs. strong for line F; and p <0.05 for none vs. mid for WT and mid vs. strong for line D. b Serum 17β-estradiol (E 2 ) levels for WT (n = 13) and line F (ERΔ3, n = 16) 3-month-old female mice in estrus were significantly different (p = 0.009, Mann–Whitney test). c Progesterone (P 4 ) serum levels were not significantly different for WT (n = 13) and line F ERΔ3 mice (n = 16) in estrus at age 3 months
Mammary Cancer Development and Progression in ERΔ3/Neu Bitransgenic Mice
Line F ERΔ3 mice were crossbred with MMTV-Neu (Neu) mice to induce mammary cancer. Compared to Neu mice, the survival curve for the percentage of bitransgenic ERΔ3/Neu mice without mammary tumors was significantly and consistently shifted to later ages until age 16 months (p = 0.0006, Gehan–Breslow–Wilcoxon; Fig. 2a). Tumor incidence was not significantly different between the genotypes, but tumor onset was significantly delayed in ERΔ3/Neu mice (p < 0.002, Mann–Whitney). The significant decrease in micrometastases incidence detected in the lung by histopathology in ERΔ3/Neu versus Neu mice (p = 0.0002, Fisher’s exact) indicates that ERΔ3 expression also inhibited tumor progression. Although the incidence of grossly detected metastatic lung lesions was also lower in ERΔ3/Neu mice, the difference was not significant (Fig. 2b). The similar time for tumor growth in each genotype (time between detection and death; Fig. 2c) indicates that the reduced metastatic incidence was due to ERΔ3 expression and not to Neu mice having more time for tumor progression. Tumor growth was also not affected since the mammary tumor doubling time was similar in ERΔ3/Neu and Neu mice (Fig. 2d). Thus, the delay in tumor onset and the lower metastatic incidence likely account for the later age of death in ERΔ3/Neu versus Neu mice (Fig. 2e).
Delay in mammary tumor development and reduced metastatic incidence in ERΔ3/Neu vs. Neu mice. a Percent of tumor-free mice with age show a significant shift to older ages for tumor detection in ERΔ3/Neu female mice (n = 77) compared to MMTV-Neu (Neu, n = 88), p = 0.0006 Gehan–Breslow–Wilcoxon test, p = 0.0016 log-rank test. b The percentage of tumor-bearing mice with lung micrometastases detected by histopathology (pathology) and visible lung lesions detected at necropsy (gross) which were confirmed by histopathology to be metastatic tumors are shown for Neu (n = 66) and ERΔ3/Neu mice (n = 55). ***p = 0.0002, Fisher’s exact test vs. Neu mice by pathology; p > 0.05, Fisher’s exact test for gross lesions. c The mean length of time between mammary tumor detection and death (days with tumor) was similar for Neu (n = 66) and ERΔ3/Neu mice (n = 55), p > 0.05, Mann–Whitney test. d Mammary tumor doubling time for mice with a single mammary tumor that was 3 mm × 4 mm or smaller at detection was calculated as described in the “Materials and Methods”. p >0.05, Mann–Whitney test for Neu (n = 17) and ERΔ3/Neu mice (n = 14). e The age of death for Neu (n = 81) and ERΔ3/Neu mice (n = 72) with and without mammary tumors was significant, p = 0.0006, Mann–Whitney test. Mice that died young without mammary tumors were excluded
Expression of the Neu transgene and endogenous Neu gene in mammary tissue was similar in Neu and ERΔ3/Neu mice (Fig. 3a). ERΔ3 transcripts were expressed at significantly higher levels than ERα (8:1 ratio) in the ERΔ3/Neu mammary gland (Fig. 3b). However, ERα RNA levels were lower in ERΔ3/Neu than Neu mice, though not significantly (Fig. 3c). Additionally, PR transcript levels were significantly increased in ERΔ3/Neu mammary tissue (Fig. 3c). Since PR immunostaining intensity in epithelial cells was decreased, an epithelial marker, cytokeratin 18 (Krt18), was examined to compare ERΔ3/Neu and Neu mammary tissue. Krt18 mRNA levels were comparable in both genotypes, suggesting a similar amount of epithelium and maturity of the mammary tissue in the 3-month-old ERΔ3 and WT mice. PR transcripts normalized to Krt18 remained significantly elevated in ERΔ3/Neu mice (Fig. 3c). Therefore, ERΔ3 reduced epithelial expression of PR protein (Fig. 1a), but increased its RNA levels in mammary tissue.
RNA levels of Neu transgene, endogenous Neu gene, ERα, ERΔ3, PR, and keratin 18 in mammary tissue. Total RNA from mammary glands of 3-month-old mice in estrus was analyzed by real-time RT–PCR. The threshold cycle (CT) for the gene of interest was normalized to the housekeeping gene, cyclophilin A (Ppia), to calculate the ΔCT values. The fold change of the black bar relative to the white bar calculated by the 2−ΔΔCt method is shown within each bar. (Lower ΔCT values reflect higher levels of expression.) a No significant differences were found by Mann–Whitney test (p > 0.05) between Neu and ERΔ3/Neu female mice for the rat Neu transgene (transgene) or mouse Neu gene (endogenous); n = 8 for both genotypes for the transgene; and n = 4 Neu and n = 3 ERΔ3/Neu for the endogenous gene. b In ERΔ3/Neu female mice, expression levels of the ERΔ3 transgene were higher than the Esr1 gene (ERα), n = 8. ***p = 0.0006, Mann–Whitney test. c Levels of progesterone receptor gene (Pgr) were significantly higher in ERΔ3/Neu mice (n = 8) compared to Neu mice (n = 8) whether it was normalized to cyclophilin (PR (cph) ; p = 0.003, Mann–Whitney) or cytokeratin 18 (PR (krt18) ; p = 0.01, Mann–Whitney). Cytokeratin 18 (krt18) is similar for the two genotypes (p > 0.05, Mann–Whitney). Levels of ERα were lower in ERΔ3/Neu mice, but the difference was not significant compared to Neu mice (p > 0.05, Mann–Whitney)
Mammary Cancer Prevention with Tamoxifen and Soy Isoflavones
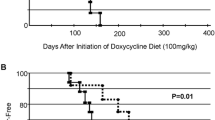
To determine if therapies with antiestrogen activity can augment ERΔ3 protection, Neu and ERΔ3/Neu mice were treated with tamoxifen (20 mg/1,800 kcal) and soy isoflavones (181 mg/1,800 kcal). In Neu mice, tumor incidence was not affected by soy, but was significantly reduced with tamoxifen compared to the control group (p < 0.0001, Fisher’s exact; Fig. 4a). Similar effects were observed in ERΔ3/Neu mice with tamoxifen suppressing tumor incidence compared to the control group (p < 0.0001, Fisher’s exact). For comparisons between the genotypes, soy had no effect; however, tamoxifen prevention was significantly augmented in ERΔ3/Neu mice since only one mouse developed a mammary tumor (1.7 %) compared to 18.6 % of Neu females (p = 0.0016, Fisher’s exact; Fig. 4a). With only one tumor-bearing ERΔ3/Neu mouse, latency for tamoxifen-treated mice could not be analyzed. For soy, tumor onset was significantly delayed in ERΔ3/Neu versus Neu mice, but the control and soy groups were not different in ERΔ3/Neu females (Fig. 4b).
Tamoxifen preventative efficacy is enhanced in ERΔ3/Neu mice, in contrast to the higher metastatic incidence in the soy-treated group. a Maximal mammary tumor incidence for control (n = 81), soy-treated (374 mg/kg diet or 181 mg/1,800 kcal; n = 78), and tamoxifen-treated (20 mg/1,800 kcal; n = 70) groups in Neu mice up to maximal age of 16 months was significant by the chi-squared test, p < 0.0001 as was these groups in ERΔ3/Neu mice (n = 72, 60, and 60, respectively), p < 0.0001, chi-squared test. For comparisons between the genotypes, the tamoxifen-treated ERΔ3/Neu mice had a significantly lower incidence (one tumor) compared to Neu mice, p = 0.0016, Fisher’s exact test; but the control and soy groups were not significantly different, p > 0.05, Fisher’s exact test. b Tumor latency occurred at significantly older ages in ERΔ3/Neu mice compared to Neu females in the control groups (p = 0.0018, Mann–Whitney; n = 77 Neu, n = 62 ERΔ3/Neu) and with soy treatment (p = 0.0012, Mann–Whitney; n = 72 Neu, n = 51 ERΔ3/Neu). One-way ANOVA analysis on the Neu mice found no significant differences (p > 0.05; n = 13 tamoxifen). No difference was detected between the control and soy groups in the ERΔ3/Neu mice (p > 0.05, Mann–Whitney); the tamoxifen group could not be analyzed with an n = 1. c Survival curves depicting the percentage of mice without tumors with age for all six groups are shown. For comparisons within each genotype, control (CON) versus tamoxifen (TAM) groups were significant for Neu and for ERΔ3/Neu mice (p < 0.0001, Gehan–Breslow–Wilcoxon and log-rank), but not for control versus soy treatment for either genotype (p > 0.05). For comparisons between the genotypes, both soy (p = 0.0004, Gehan–Breslow–Wilcoxon; p = 0.0061, log-rank) and tamoxifen treatments (p = 0.0019, Gehan–Breslow–Wilcoxon; p = 0.0017 log-rank) were significant for Neu (n = 81, SOY; n = 73, TAM) versus ERΔ3/Neu (n = 62, SOY; n = 66, TAM) female mice. Differences between the control groups are listed in Fig. 2a. d Tumor multiplicity was significant only for the tamoxifen group compared to the control and soy groups in Neu mice (p = 0.0029, one-way ANOVA; p < 0.05 control vs. tamoxifen and p < 0.01 soy vs. tamoxifen, Tukey’s test; n = 77 control, n = 72 soy, n = 13 tamoxifen). No significant differences were detected between the control (n = 63) and soy (n = 51) in the ERΔ3/Neu mice (p > 0.05, t test; tamoxifen could not be analyzed, n = 1). e Incidence of metastatic cancer in the lungs of tumor-bearing mice detected by histopathology in the tamoxifen-treated Neu females (n = 13) versus the control group (n = 66; p < 0.005, Fisher’s exact test) and the soy-treated mice (n = 61; p < 0.013, Fisher’s exact test) was significantly different, but was similar for the control and soy-treated Neu mice. The incidence was significantly higher in the soy-treated ERΔ3/Neu mice (n = 47) compared to the control group (n = 55; p = 0.0025, Fisher’s exact test); tamoxifen could not be analyzed, n = 1. The soy groups in ERΔ3/Neu versus Neu mice were not significant. Differences in the control groups are described in Fig. 2b. f The mean number of metastatic lesions/mouse detected in the lungs of tumor-bearing mice analyzed by histopathology is shown for the six treatment groups. Comparisons between the Neu groups was not significant by one-way ANOVA, but control and tamoxifen groups were significant by Mann–Whitney test, p = 0.011. In the ERΔ3/Neu mice, control mice had significantly fewer micrometastases/mouse compared to soy-treated animals (p < 0.003, Mann–Whitney); tamoxifen group could not be analyzed (eight micrometastases detected in the only tumor-bearing mouse). The ERΔ3/Neu control group had significantly fewer micrometastases/mouse compared to Neu mice (p = 0.0013, Mann–Whitney), but the soy groups were similar [n per group are listed in panel (e)]. a Significant vs. control; b significant vs. soy; *p < 0.05, **p < 0.01, and ***p < 0.001 for ERΔ3/Neu vs. Neu mice (same treatment)
The tamoxifen survival curves were significantly different from the control group within each genotype (p < 0.0001, Gehan–Breslow–Wilcoxon for both genotypes; Fig. 4c). The ERΔ3/Neu curve illustrates the near complete prevention with tamoxifen, which was statistically significant compared to tamoxifen-treated Neu mice (p = 0.0019, Gehan–Breslow–Wilcoxon). With soy treatment, the ERΔ3/Neu curve was significantly shifted to later ages than for Neu mice (p = 0.0004, Gehan–Breslow–Wilcoxon). However, compared to the control groups, both the soy-treated Neu and ERΔ3/Neu curves shifted toward earlier ages in the middle of the curves, but no significant difference was detected (Fig. 4c). Tamoxifen reduced tumor multiplicity in Neu mice (cannot be analyzed in ERΔ3/Neu mice); whereas, soy had no effect (Fig. 4d). These data indicate that soy did not modify tumor development in Neu and ERΔ3/Neu mice, but the strong tamoxifen protection was enhanced in mice expressing ERΔ3.
In Neu mice, tamoxifen significantly reduced the incidence of micrometastases compared to the control group (p < 0.005, Fisher’s exact; Fig. 4e). For Neu mice, metastatic incidence in the soy-treated group was similar to the Neu control group and to soy-treated ERΔ3/Neu mice. In contrast, the reduced incidence of metastatic lung lesions in ERΔ3/Neu versus Neu mice was lost with soy treatment (Fig. 4e) since the ERΔ3/Neu control group was significantly lower than the soy group (p = 0.0025, Fisher’s exact). No significant differences were detected in the time with tumor between the groups (data not shown). Therefore, at the tested dose, soy treatment reversed ERΔ3 protection on metastatic progression.
The number of metastatic lesions per tumor-bearing mouse detected by histopathology was lower in Neu animals treated with tamoxifen (p < 0.012, Mann–Whitney) and soy, compared to the control group, but the difference with soy was not significant (Fig. 4f). In ERΔ3/Neu mice, soy-treated mice had significantly more lung micrometastases than the control group (p < 0.003, Mann–Whitney). Comparisons between Neu and ERΔ3/Neu mice detected that the soy groups were not significantly different, unlike the control groups (p < 0.0015, Mann–Whitney). These results correlate with the metastatic incidence in these groups (Fig. 4e), except for the non-significant reduction in micrometastases/mouse observed in soy-treated Neu mice.
Effects of Soy Isoflavones in Ovariectomized Neu Mice
Tumor development was examined in ovariectomized (OVX) Neu mice with and without soy treatment to test for potential estrogenic stimulation by these phytoestrogens in an estrogen-deficient environment. Although tumor incidence was significantly reduced compare to intact animals, no difference was detected between OVX control and soy-treated Neu mice (Fig. 5a). Tumor latency was also not affected (Fig. 5b) and uterine weight was not stimulated by the estrogenic isoflavones (Fig. 5c). Incidence of metastatic lesions detected by histopathology was also non-significant for the control (29 %, n = 7) and soy-treated (13 %, n = 8) OVX Neu mice.
Treatment with soy protein isolate with isoflavones did not affect mammary tumor incidence or latency or uterine weight in ovariectomized Neu mice. a The incidence in mammary tumors in intact (n = 81 control, n = 78 soy) and ovariectomized (OVX; n = 33 control, n = 41 soy) mice at maximum age of 13.5 months is shown. In OVX mice, the incidence is similar for the control and soy groups. A significantly lower incidence was detected in OVX vs. intact Neu mice. ***p < 0.0001, Fisher’s exact test for intact vs. OVX mice (same treatment). b Mammary tumor latency was not different between the intact and OVX groups for either treatment group up to the maximum age of 13.5 months (p > 0.05, two-way ANOVA; n = 73 intact/control, n = 66 intact/soy, n = 7 OVX/control, n = 9 OVX/soy). c Uterine wet weight normalized to body weight was similar for OVX Neu mice in the control and soy groups (p > 0.05, Mann–Whitney). Body weight was also not significant (data not shown)
Uterine and Body Weights in ERΔ3/Neu and Neu Mice
Uterine wet weight in intact tumor study mice in diestrus was analyzed to determine genotype and treatment effects (Fig. 6a). Tamoxifen significantly reduced uterine weight normalized to body weight (BW) in both genoytpes versus their control group. Uterine weight/BW in tamoxifen-treated ERΔ3/Neu was significant compared to Neu mice (p = 0.007, Mann–Whitney). In tamoxifen-treated mice, body weight was also significantly lower compared to control mice for each genotype (Fig. 6b) and ERΔ3/Neu mice were significantly smaller than Neu females (p < 0.0001, Mann–Whitney). Soy treatment did not influence uterine weight or body weight. Therefore, as with the cancer outcomes, tamoxifen effects were modified by ERΔ3 expression.
Tamoxifen treatment reduces uterine wet weight and body weight in ERΔ3/Neu and Neu female mice. a For mice in diestrus at necropsy, tamoxifen reduced uterine weight (Ut wt) normalized to body weight (BW) in Neu and ERΔ3/Neu mice compared to the control and soy groups within each genotype (two-way ANOVA, p < 0.0001 for the treatments, not significant for genotype or interaction) (n = 49 control, n = 55 soy, n = 55 tamoxifen for Neu mice; n = 40 control, n = 41 soy, n = 49 tamoxifen for ERΔ3/Neu mice). a Significant by Bonferroni vs. control, p < 0.001; b significant by Bonferroni test versus soy, p < 0.001; **p=0.007 for ERΔ3/Neu vs. Neu mice (same treatment). b Body weights (BW) at death were lower in tamoxifen-treated mice compared to control and soy-treated mice for each genotype (two-way ANOVA, p = 0.0005 for genotype, p < 0.0001 for treatment and the interaction). With tamoxifen treatment, ERΔ3/Neu mice were significantly smaller than Neu mice (***p < 0.001, Bonferroni test); however, control and soy groups were similar between the genotypes (n = 80 control, n = 75 soy, n = 71 tamoxifen for Neu mice; n = 69 control, n = 60 soy, n = 58 tamoxifen for ERΔ3/Neu mice). a Significant by Bonferroni vs. control, p<0.001; b significant by Bonferroni vs. soy, p<0.01
Discussion
ERΔ3 Effects on Mammary Tumor Development
This study provides the first evidence that an ERα variant influences mammary tumor development. As predicted, ERΔ3 expression protected against Neu-induced cancer. The similar expression of the Neu transgene in Neu and ERΔ3/Neu mice verifies that ERΔ3 expression does not affect the MMTV promoter. Therefore, the delayed tumor formation is related to ERΔ3 actions and not to model-specific effects on Neu transgene expression. Based on the known roles of estrogen in breast cancer [29], the later tumor onset suggests that ERΔ3 suppressed estrogen action in the mammary gland, which correlates with its reported, in vitro dominant negative activity [6, 7]. Although the MMTV-Neu mice develop estrogen-independent tumors that mimic HER2/Neu breast cancer, tumor development requires estrogen, as was observed in tamoxifen-treated and estrogen-deficient Neu mice in this (Figs. 4a and 5a) and previous studies [14–18]. Therefore, ERΔ3 would likely be protective in women and in other preclinical models of breast cancer with estrogen-dependent tumorigenesis.
The delayed tumor onset suggests that ERΔ3 expression in normal mammary tissue influences determining events involved in cancer development. Generally, ERα repressors must be in excess of the WT receptor for dominant negative activity [1], as was detected in the ERΔ3/Neu mammary gland for ERΔ3 relative to ERα (8:1 ratio; Fig. 3b). However, in our preliminary analysis of this transgenic model, ERΔ3 transcripts were less prevalent than ERα in the mammary gland of a line F female mouse (0.6:1 ratio) [11]. The variation between the two studies may be due to inter-individual expression differences in ERα as well as ERΔ3, to the co-expression of Neu in the ERΔ3/Neu mice, or to evaluation of mice in estrus for this study, unlike in the previous study in which the cycle stage was not determined. In the rat uterus, alternative splicing transcripts of ERΔ3 are increased during proestrus and estrus (when E2 levels are high) compared to diestrus [30]. Since E2 upregulates ERα transcripts levels by stabilizing its mRNA [31], cycle stage may modify message stability of ERα and/or the ERΔ3 transgene, thereby affecting the ERΔ3:ERα ratios.
In women, ERΔ3 transcripts are common in normal breast tissue [8, 9, 32–34]. One study compared ERΔ3 and ERα transcript levels from reduction mammoplasties; ERΔ3 was expressed at higher levels in mammary epithelial cells with ratios ranging between 0.4 and 9.8:1 for ERΔ3:ERα [8]. This ratio range correlates with ratios detected in the mammary glands of ERΔ3 transgenic mice (0.6:1 and 8.4:1). The similar ratio range in both species suggests ERΔ3 has potential to inhibit ERα action and, possibly, provide similar anticancer protection in women. Accordingly, the delayed tumor onset in ERΔ3/Neu mice could mean women expressing ERΔ3 may develop breast cancer later and/or be less likely to develop early-onset breast cancer. Based on the prolonged latency without changes in tumor incidence in ERΔ3/Neu mice, future studies will need to correlate ERΔ3 expression in the normal breast with tumor onset; however, effects on breast cancer incidence would not be expected.
In ERΔ3 mice, the higher serum E2 levels would increase the amount of local estrogen available to stimulate the mammary tissue, but ERΔ3 should suppress its actions. The modified E2 levels are likely related to ERΔ3 expression in non-mammary tissues and may not occur in women. Although ERΔ3 has been detected in human pituitary adenomas [35], its expression is not common or at the levels observed in normal breast tissue [8, 9, 32–34]. However, elevated estrogen levels could occur in women due to other causes or therapies. Thus, tumor suppression in the mice suggests that even with elevated E2 concentrations and, possibly with other natural or synthetic estrogens, estrogen activity may be mitigated in mammary tissue expressing ERΔ3, unlike in glands without ERΔ3. Therefore, expression of ERΔ3 in normal breast tissue may be cancer protective even in women taking estrogen therapies or producing more estrogen, locally or systemically.
Delayed mammary cancer onset suggests ERΔ3 affects tumor promotion, a stage of carcinogenesis influenced by hormones. Since estrogen is required for ERΔ3 to dimerize with WT ER and inhibit its actions, the elevated E2 levels may inhibit versus stimulate tumor promotion through ERΔ3:ERα and ERΔ3:ERβ heterodimers. P4 effects may also be reduced in mice expressing ERΔ3 due to decreased PR expression in the mammary epithelium (Fig. 1a). Mammary epithelial proliferation is highest in the secretory (luteal) phase of the estrous cycle when P4 levels peak [36, 37]; therefore, reducing the stimulatory actions of P4 by reducing its receptor expression may also contribute to the delayed tumor onset in ERΔ3/Neu mice.
Although immunostaining intensity was reduced in ERΔ3 mice, PR transcripts were increased in ERΔ3/Neu versus Neu mice. Since PR immunostaining was only examined in the mammary epithelium, the increased PR RNA expression could be due to its levels in non-epithelial cells or to post-transcriptional effects reducing epithelial receptor levels. Pgr RNA levels are likely increased in ERΔ3/Neu mammary tissue through non-classical mechanisms. The PGR gene does not contain an ERE; instead estrogen regulation occurs through non-classical signaling on AP-1, Sp1, and Sp1/half-ERE sites in its promoter [38–40]. Human and mouse ERα missing the second zinc finger stimulate expression of an Sp1–reporter [10] and human ERΔ3 activates transcription of an AP-1/half-ERE reporter [7]. However, in transfected MCF-7 cells, ERΔ3 suppressed expression of pS2, a gene with several imperfect EREs [8]. Therefore, the loss of the second zinc finger likely inhibits endogenous genes containing EREs, as shown previously with an ERE–reporter [6]; however, ERΔ3 should stimulate genes regulated by non-classical mechanisms, such as Pgr.
Cancer protection in ERΔ3/Neu mice and ERΔ3’s ability to activate non-classical pathways [7, 10] suggest that non-classical ER signaling does not stimulate mammary tissue. This concept is in accord with the diminished mammary gland differentiation in untreated and P4-treated NERKI females [41]. NERKI mice express an ERα receptor with a mutation in the first zinc finger of the DBD that prevents classic ERE stimulation, but retains non-classical signaling activity [41]. Despite differences to the ERΔ3 model, including that heterozygous females in the knock-in NERKI model are infertile, anovulatory, and have decreased serum P4 levels and the mutant does not have dominant negative activity, both models express WT ERα and a non-classical-specific ERα receptor and have inhibitory actions in mammary tissue. In contrast, non-classical ER signaling appears to stimulate the uterus since NERKI uteri are hypersensitive to estrogen and exhibit cystic endometrial hyperplasia [41] and ERΔ3 expression accelerates neonatal DES-induced uterine cancer [11]. These data also correlate with tamoxifen, which has similar opposing actions in the uterus and mammary glands and stimulates non-classical ER pathways [42, 43].
Tumor growth was not affected by ERΔ3 expression, as might be expected for a model with estrogen-independent mammary tumors. In vitro, expression of ERΔ3 inhibits proliferation of estrogen-responsive MCF-7 cells [8], but the in vivo effects of ERΔ3 on estrogen-dependent breast tumor growth remain untested. In contrast, metastatic incidence and burden were substantially reduced in ERΔ3/Neu mice. Possibly, ERΔ3 suppresses tumor aggressiveness prior to estrogen-independence or it has actions in the absence of WT ERα. In estrogen-responsive, stably transfected MCF-7 cells, ERΔ3 diminished their ability to grow in soft agar and invade chick embryo chorioallantoic membranes compared to the parental cells [8]; these attenuated phenotypes correlate with the lower metastatic incidence in ERΔ3/Neu mice. These findings suggest that women expressing ERΔ3 in the breast or in estrogen-dependent and -independent breast tumors may be at reduced risk for metastatic breast cancer.
Since other ERα variants are common in the breast, it is unknown how ERΔ3 may act in their presence. However, ERΔ3 should not dimerize with ERΔ2, ERΔ5, or ERΔ7 variants, which do not have the ligand-dependent dimerization domain, or ERΔ4, which would not localized to the nucleus [1]. Similarly, ERΔ3 may not interact with ERβ variants missing these essential domains.
Although ERΔ3 expression delayed mammary tumor formation, it does not affect normal reproductive functions, such as fertility and lactation. Although correlations between ERΔ3 and dysfunctions in human reproductive responses have not been explored, based on the lack of effects in the mice, breast function would likely be unaffected. Therefore, expression of this variant may provide breast cancer protection without adverse effects, such as those associated with preventative therapies, like tamoxifen.
Tamoxifen Prevention in ERΔ3/Neu Mice
Tamoxifen chemoprevention in Neu mice was similar to previous reports for this model [16, 17]. Its ability to inhibit tumorigenesis is probably related to starting treatment prior to the initiated tumors becoming estrogen independent. In mice expressing ERΔ3, the superior chemoprevention may be due to enhanced estrogen inhibition with tamoxifen bound to ERΔ3 and/or the delay in tumor onset in ERΔ3/Neu mice, which would allow fewer estrogen-resistant neoplastic lesions to form prior to starting tamoxifen treatment. With either mechanism, these findings suggest that tamoxifen may be more efficacious for preventing breast cancer in women expressing ERΔ3 in the pre-neoplastic breast. If the delayed onset contributes to the enhanced protection, women expressing ERΔ3 in breast tissue may be able to start tamoxifen at later ages without reducing its preventative capability.
Tamoxifen acts via non-classical signaling [42, 43] and inhibits mammary cancer in Neu mice and women [14–17, 20]. ERΔ3 cannot induce classical ERE signaling [6], and tamoxifen or E2 bound to the mouse and human ERα variant lacking the second zinc finger stimulates non-classical signaling [10]. Therefore, tamoxifen bound to ERΔ3 likely acts via non-classical ER pathways to enhance cancer prevention. With this increased anticancer efficacy, perhaps lower tamoxifen doses could provide sufficient protection with fewer adverse events, which may encourage more at-risk women to use this therapy. Since identifying subpopulations with improved outcomes is a desirable goal, the mouse results suggest further studies may optimize tamoxifen prevention for women expressing ERΔ3 in normal breast tissue.
Expression of variants in breast cancer has been suggested to contribute to tamoxifen resistance, but an MCF-7 variant transfected with ERΔ3 retained tamoxifen sensitivity [5]. Due to formation of only one tumor in ERΔ3/Neu mice, ERΔ3 effects on tamoxifen responsiveness cannot be determined. However, its inhibitory actions on primary and metastatic tumor development in control and tamoxifen-treated ERΔ3/Neu mice suggests ERΔ3 would augment versus circumvent tamoxifen’s repression of estrogen-dependent breast tumors in animals and women.
Soy Effects on Tumorigenesis in ERΔ3/Neu Mice
Unlike tamoxifen, isoflavones did not modify ERΔ3’s anticancer effects in intact females or exhibit estrogenic effects on mammary tumorigenesis or uterine weight in OVX Neu mice. In other studies treating Neu mice with isoflavone-rich soy protein after puberty, mammary tumor onset was delayed [16, 17, 23], which could be related to dose effects as our dose was approximately 70 % lower than their doses. However, another critical difference is that ERΔ3/Neu and Neu mice were not exposed to isoflavones from conception until 2 months of age, unlike the other studies using mice raised on soy-based chow [16, 17, 23]. Since developmental through adult exposure to isoflavones is protective for mammary carcinogenesis [44, 45], starting exposure in adult Neu and ERΔ3/Neu females may be related to the unmodified latencies versus control mice. These data also fit with studies showing breast cancer protection in Asian women that consume soy throughout their life in contrast to supplementing Western diets late in life [25].
In orthotopic breast cancer models, genistein [46], soy protein with isoflavones [47], and isoflavone-depleted soy protein reduced metastatic burden [48]. In Neu mice, metastatic burden was also reduced in soy-treated mice compared to the control group, but it was not significant. However, metastatic incidence was unaffected by exposure to isoflavone-rich soy protein.
In ERΔ3/Neu mice, the loss of metastatic cancer protection suggests soy isoflavones counteract the beneficial actions of ERΔ3, which may be related to inhibition of ERE-regulated genes, heterodimerization with ERβ, and/or non-classical signaling. For example, since genistein and daidzein bind weakly to ERα [49], soy isoflavones may be less effective at activating ERΔ3 dominant negative activity on ERE-containing genes. Additionally, as genistein and daidzein bind preferentially to ERβ [49], the loss of metastatic protection in the soy-treated ERΔ3/Neu mice may be related to soy isoflavone-induced dimerization of ERΔ3 with ERβ versus ERα. These data may suggest that ERβ-selective ligands may not provide the same protection as ERα-selective ligands in breast tissue expressing ERΔ3. However, these data are incongruent with reports that ERβ overexpression in breast cancer xenografts stimulates metastasis [50] and ERβ-positive breast tumors are associated with a poor prognosis [51, 52], as inhibition of ERβ action might be predicted to be protective. For non-classical signaling, genistein and daidzein also upregulate an Sp1–reporter construct via ERα; however, high doses are required to activate the reporter in contrast to stronger stimulation with lower doses of E2 and tamoxifen [53]. Additionally, genistein and daidzein inhibit AP-1 activity [54, 55], unlike E2 and tamoxifen [42]. Therefore, isoflavones may have dissimilar effects on ERΔ3 non-classical signaling than tamoxifen or E2, both of which suppressed metastatic incidence and burden.
The delayed tumor onset in ERΔ3/Neu mice suggests that ERΔ3 expression in the normal breast may provide women with similar protection. The inhibition of estrogen action in the breast is a central issue to the prevention and treatment of breast cancer; however, estrogen provides beneficial effects in other systems, such as cardiovascular, skeletal, and reproductive tissues. Therefore, the ability of ERΔ3 to inhibit estrogen-regulated mechanisms in the mammary gland without suppressing circulating estrogen levels or its actions in other estrogen-responsive tissues would be advantageous for the prevention of breast cancer as well as to a woman’s quality of life. The contrasting effects of tamoxifen and soy isoflavones highlight that different estrogens may have varying effects on ERΔ3 actions. Therefore, exposure to different estrogens (i.e., environmental, dietary, synthetic, and endogenous estrogens) throughout a woman’s lifetime may affect the level of cancer protection provided by ERΔ3.
References
Herynk MH, Fuqua SAW (2004) Estrogen receptor mutations in human disease. Endocr Rev 25:869–898
Desai AJ, Luqmani YA, Walters JE, Coope RC, Dagg B, Gomm JJ, Pace PE, Rees CN, Thirunavukkarasu V, Shousha S, Groome NP, Coombes R, Ali S (1997) Presence of exon 5-deleted oestrogen receptor in human breast cancer: functional analysis and clinical significance. Br J Cancer 75:1173–1184
Park W, Choi JJ, Hwang ES, Lee JH (1996) Identification of a variant estrogen receptor lacking exon 4 and its coexpression with wild-type estrogen receptor in ovarian carcinomas. Clin Cancer Res 2:2029–2035
Fasco MJ, Amin A, Pentecost BT, Yang Y, Gierthy JF (2003) Phenotypic changes in MCF-7 cells during prolonged exposure to tamoxifen. Mol Cell Endocrinol 206:33–47
Han F, Miksicek R, Clarke R, Conrad SE (2004) Expression of an estrogen receptor variant lacking exon 3 in derivatives of MCF-7 cells with acquired estrogen independence or tamoxifen resistance. J Mol Endocrinol 32:935–945
Wang Y, Miksicek RJ (1991) Identification of a dominant negative form of the human estrogen receptor. Mol Endocrinol 5:1707–1715
Bollig A, Miksicek RJ (2000) An estrogen receptor-alpha splicing variant mediates both positive and negative effects on gene transcription. Mol Endocrinol 14:634–649
Erenburg I, Schachter B, Mira y Lopez R, Ossowski L (1997) Loss of an estrogen receptor isoform (ER alpha delta 3) in breast cancer and the consequences of its reexpression: interference with estrogen-stimulated properties of malignant transformation. Mol Endocrinol 11:2004–2015
Leygue E, Dotzlaw H, Watson PH, Murphy LC (2000) Altered expression of estrogen receptor-alpha variant messenger RNAs between adjacent normal breast and breast tumor tissues. Breast Cancer Res 2:64–72
Kim K, Thu N, Saville B, Safe S (2003) Domains of estrogen receptor alpha (ERalpha) required for ERalpha/Sp1-mediated activation of GC-rich promoters by estrogens and antiestrogens in breast cancer cells. Mol Endocrinol 17:804–817
Davis VL, Newbold RR, Couse JF, Rea SL, Gallagher KM, Goulding EH, Jefferson W, Eddy EM, Bullock BC, Korach KS (2012) Expression of a dominant negative estrogen receptor alpha variant in transgenic mice accelerates uterine cancer induced by the potent estrogen diethylstilbestrol. Reprod Toxicol doi:10.1016/j.reprotox.2012.08.005
Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ (1992) Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A 89:10578–10582
Cardiff RD, Wellings SR (1999) The comparative pathology of human and mouse mammary glands. J Mammary Gland Biol Neoplasia 4:105–122
Menard S, Aiello P, Tagliabue E, Rumio C, Lollini PL, Colnaghi MI, Balsari A (2000) Tamoxifen chemoprevention of a hormone-independent tumor in the proto-neu transgenic mice model. Cancer Res 60:273–275
Nanni P, Nicoletti G, De Giovanni C, Landuzzi L, Di Carlo E, Iezzi M, Ricci C, Astolfi A, Croci S, Marangoni F, Musiani P, Forni G, Lollini P-L (2003) Prevention of HER-2/neu transgenic mammary carcinoma by tamoxifen plus interleukin 12. Int J Cancer 105:384–389
Liu B, Edgerton S, Yang X, Kim A, Ordonez-Ercan D, Mason T, Alvarez K, McKimmey C, Liu N, Thor A (2005) Low-dose dietary phytoestrogen abrogates tamoxifen-associated mammary tumor prevention. Cancer Res 65:879–886
Yang X, Edgerton SM, Kosanke SD, Mason TL, Alvarez KM, Liu N, Chatterton RT, Liu B, Wang Q, Kim A, Murthy S, Thor AD (2003) Hormonal and dietary modulation of mammary carcinogenesis in mouse mammary tumor virus-c-erbB-2 transgenic mice. Cancer Res 63:2425–2433
Landis MD, Seachrist DD, Abdul-Karim FW, Keri RA (2006) Sustained trophism of the mammary gland is sufficient to accelerate and synchronize development of ErbB2/Neu-induced tumors. Oncogene 25:3325–3334
Kumar V, Chambon P (1988) The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell 55:145–156
Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, Boyle P (2003) Overview of the main outcomes in breast-cancer prevention trials. Lancet 361:296–300
Messina M, McCaskill-Stevens W, Lampe JW (2006) Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst 98:1275–1284
Bouker KB, Hilakivi-Clarke L (2000) Genistein: does it prevent or promote breast cancer? Environ Health Perspect 108:701–708
Jin Z, MacDonald RS (2002) Soy isoflavones increase latency of spontaneous mammary tumors in mice. J Nutr 132:3186–3190
Kinsinger LS, Harris R, Woolf SH, Sox HC, Lohr KN (2002) Chemoprevention of breast cancer: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 137:59–69
Nagata C (2010) Factors to consider in the association between soy isoflavone intake and breast cancer risk. J Epidemiol 20:83–89
Setchell KDR, Brown NM, Zhao X, Lindley SL, Heubi JE, King EC, Messina MJ (2011) Soy isoflavone phase II metabolism differs between rodents and humans: implications for the effect on breast cancer risk. Am J Clin Nutr 94:1284–1294
Davis VL, Jayo MJ, Ho A, Kotlarczyk MP, Hardy ML, Foster WG, Hughes CL (2008) Black cohosh increases metastatic mammary cancer in transgenic mice expressing c-erbB2. Cancer Res 68:8377–8383
Lindholm J, van Diest PJ, Haffner D, Mikuz G, Wegner AR (1992) A morphometric filter improves the diagnostic value of morphometric analyses of frozen histopathological sections from mammary tumours. Anal Cell Pathol 4:443‑449
Parsa P, Parsa B (2009) Effects of reproductive factors on risk of breast cancer: a literature review. Asian Pac J Cancer Prev 10:545–550
Varayoud J, Ramos JG, Monje L, Bosquiazzo V, Munoz-de-Toro M, Luque EH (2005) The estrogen receptor alpha sigma3 mRNA splicing variant is differentially regulated by estrogen and progesterone in the rat uterus. J Endocrinol 186:51–60
Mitchell DC, Ing NH (2003) Estradiol stabilizes estrogen receptor messenger ribonucleic acid in sheep endometrium via discrete sequence elements in its 3′-untranslated region. Mol Endocrinol 17:562–574
Leygue ER, Watson PH, Murphy LC (1996) Estrogen receptor variants in normal human mammary tissue. J Natl Cancer Inst 88:284–290
Chappell SA, Johnson SM, Shaw JA, Walker RA (2000) Expression of oestrogen receptor alpha variants in non-malignant breast and early invasive breast carcinomas. J Pathol 192:159–165
van Dijk MA, Hart AA, van ’t Veer LJ (2000) Differences in estrogen receptor alpha variant messenger RNAs between normal human breast tissue and primary breast carcinomas. Cancer Res 60:530–533
Chaidarun SS, Klibanski A, Alexander JM (1997) Tumor-specific expression of alternatively spliced estrogen receptor messenger ribonucleic acid variants in human pituitary adenomas. J Clin Endocrinol Metab 82:1058–1065
Fata JE, Chaudhary V, Khokha R (2001) Cellular turnover in the mammary gland is correlated with systemic levels of progesterone and not 17beta-estradiol during the estrous cycle. Biol Reprod 65:680–688
Going JJ, Anderson TJ, Battersby S, MacIntyre CC (1988) Proliferative and secretory activity in human breast during natural and artificial menstrual cycles. Am J Pathol 130:193–204
Petz LN, Nardulli AM (2000) Sp1 binding sites and an estrogen response element half-site are involved in regulation of the human progesterone receptor A promoter. Mol Endocrinol 14:972–985
Petz LN, Ziegler YS, Loven MA, Nardulli AM (2002) Estrogen receptor alpha and activating protein-1 mediate estrogen responsiveness of the progesterone receptor gene in MCF-7 breast cancer cells. Endocrinology 143:4583–4591
Schultz JR, Petz LN, Nardulli AM (2005) Cell- and ligand-specific regulation of promoters containing activator protein-1 and Sp1 sites by estrogen receptors alpha and beta. J Biol Chem 280:347–354
Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL (2002) An estrogen receptor (ER)alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol 16:2188–2201
Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS (1997) Differential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science 277:1508–1510
Webb P, Lopez GN, Uht RM, Kushner PJ (1995) Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol 9:443–456
Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A (2002) Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr 132:552S–558S
Warri A, Saarinen NM, Makela S, Hilakivi-Clarke L (2008) The role of early life genistein exposures in modifying breast cancer risk. Br J Cancer 98:1485–1493
Vantyghem SA, Wilson SM, Postenka CO, Al-Katib W, Tuck AB, Chambers AF (2005) Dietary genistein reduces metastasis in a postsurgical orthotopic breast cancer model. Cancer Res 65:3396–3403
Yan L, Li D, Yee JA (2002) Dietary supplementation with isolated soy protein reduces metastasis of mammary carcinoma cells in mice. Clin Exp Metastasis 19:535–540
Chiesa G, Rigamonti E, Lovati MR, Disconzi E, Soldati S, Sacco MG, Cato EM, Patton V, Scanziani E, Vezzoni P, Arnoldi A, Locati D, Sirtori CR (2008) Reduced mammary tumor progression in a transgenic mouse model fed an isoflavone-poor soy protein concentrate. Mol Nutr Food Res 52:1121–1129
Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA (1998) Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 139:4252–4263
Hou Y-F, Yuan S-T, Li H-C, Wu J, Lu J-S, Liu G, Lu L-J, Shen Z-Z, Ding J, Shao Z-M (2004) ERbeta exerts multiple stimulative effects on human breast carcinoma cells. Oncogene 23:5799–5806
Markey GC, Cullen R, Diggin P, Hill ADK, Mc Dermott EW, O’Higgins NJ, Duffy MJ (2009) Estrogen receptor-beta mRNA is associated with adverse outcome in patients with breast cancer. Tumour Biol 30:171–175
Qui W-S, Yue L, Ding A-P, Sun J, Yao Y, Shen Z, Fan L-H (2009) Co-expression of ER-beta and HER2 associated with poorer prognosis in primary breast cancer. Clin Invest Med 32:E250–260
Salvatori L, Pallante P, Ravenna L, Chinzari P, Frati L, Russo MA, Petrangeli E (2003) Oestrogens and selective oestrogen receptor (ER) modulators regulate EGF receptor gene expression through human ER alpha and beta subtypes via an Sp1 site. Oncogene 22:4875–4881
Dampier K, Hudson EA, Howells LM, Manson MM, Walker RA, Gescher A (2001) Differences between human breast cell lines in susceptibility towards growth inhibition by genistein. Br J Cancer 85:618–624
Lau TY, Leung LK (2006) Soya isoflavones suppress phorbol 12-myristate 13-acetate-induced COX-2 expression in MCF-7 cells. Br J Nutr 96:169–176
Acknowledgments
We are grateful to Protein Technologies International for providing the soy protein isolate. This work was supported by the California Breast Cancer Research Program (grant 5JB-0118 to VLD) and Wake Forest University School of Medicine Venture Grant (to JMC).
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 165 kb)
Rights and permissions
About this article
Cite this article
Davis, V.L., Shaikh, F., Gallagher, K.M. et al. Inhibition of Neu-Induced Mammary Carcinogenesis in Transgenic Mice Expressing ERΔ3, a Dominant Negative Estrogen Receptor α Variant. HORM CANC 3, 227–239 (2012). https://doi.org/10.1007/s12672-012-0122-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12672-012-0122-x