Abstract
Coronavirus infection can have various degrees of severity and outcomes. In some cases, it causes excessive production of pro-inflammatory cytokines, a so-called cytokine storm, leading to acute respiratory distress syndrome. Unfortunately, the exact pathophysiology and treatment, especially for severe cases of COVID-19, are still uncertain. Results of preliminary studies showed that immunosuppressive therapy, such as interleukin (IL)-6, IL-1, and TNF-α antagonists commonly used in rheumatology, can be considered as treatment options for COVID-19, especially in severe cases. The review focused on the most common and currently studied monoclonal antibody drugs, as well as up-to-date data on the pathogenesis of COVID-19, host immune response against SARS-CoV-2 and its association with cytokine storm. It also covered effects of interleukin (IL)-6, IL-1, and TNF-α blockers on the course of coronavirus infection and outcome in patients treated for the main autoimmune disease and subsequently infected with COVID-19.
Similar content being viewed by others
1 Background
A few important problems associated with the COVID-19 pandemic were not only a treatment of acute patients with severe complications, but also post-COVID conditions in the aftermath. An innovative approach in the use of monoclonal antibody (mAB) drugs is needed, as up to date an exact and most effective treatment for COVID-19 patients has not yet been developed. It has been shown that a systemic hyperinflammation is often observed with the development of “cytokine storm” and the formation of acute respiratory distress syndrome (ARDS) in COVID-19 patients [1]. However, the exact pathophysiological mechanisms and treatment, especially of severe COVID-19, have not yet been determined. The results of preliminary studies have shown that interleukin (IL)-6, IL-1ß, tumor necrosis factor-α (TNF-α), interferon-γ, and other cytokines play a key role in the formation of cytokine storm in patients with COVID-19 [2, 3]. Therefore, immunosuppressive therapies, such as interleukin antagonists IL-6, IL-1, and TNF-α, commonly used in rheumatology, can be considered as treatment options for COVID-19, especially in severe forms of the disease [4].
2 Cytokine Storm: Pathophysiology and Clinical Manifestation
Cytokine storm is an extremely serious condition associated with the development of uncontrolled systemic inflammation and subsequent hyperproduction of cytokines [5]. The main pathophysiological mechanism of cytokine storm development is considered to be the deregulation of a T cell activation, in which cytokines IL-6 and IFN-γ seem to play the key role. Whether a cytokine storm is the result of an anomaly of innate immune system or whether it is pathology of an adaptive immunity remains to be seen [6].
The term “cytokine storm” was first mentioned in 1993 in an article describing the graft-versus-host reaction [7]. In the following years, more new studies associated development of cytokine storm with the cytomegalovirus infection (CMV) [8], Epstein-Barr virus–associated hemophagocytic lymphohistiocytosis [9], group A streptococcus [10], and finally SARS-CoV-2, which is the etiological cause of the 2019 coronavirus pandemic [11].
Coronavirus infection of 2019 (COVID-19) is characterized by a high mortality rate. This is mainly due to the inadequate immune response in patients with the subsequent development of acute respiratory distress syndrome and multiple organ failure [12].
Already in the earliest stages of the pandemic, doctors faced an important question: what was the pathophysiological basis for the development of a critical condition associated with acute respiratory distress syndrome and multiple organ failure in certain patients? A study conducted in Wuhan led by Huang et al. proved that patients with severe COVID-19 had significantly increased levels of proinflammatory cytokines (IL-1α, IL-1β, IL-6, IL-18, and TNF-α) compared with patients with a milder course of the disease [13]. Later studies led by Fang et al. [14] and Chen et al. [15] also noted an increase in the levels of proinflammatory cytokines in patients with severe COVID-19, in particular of IL-6, which drew attention to itself, and later was recognized as a key molecule in the development of cytokine storm in patients with COVID-19 [16]. A major retrospective study led by Zhou et al. also reported that high level of IL-6 was statistically significantly correlated with patients’ mortality [17]. Therefore, drugs aimed at blocking cytokines responsible for the development of cytokine storm, in particular IL-6, could potentially be extremely successful in the complex therapy of COVID-19 [16].
2.1 Pathophysiology of Cytokine Storm
Cellular and molecular mechanisms of cytokine storm are yet not fully understood; however, there are a few reliable studies available. For example, Mahmudpour M. et al. described several molecular pathways involved in the development of cytokine storm:
-
1.
Angiotensin-converting enzyme/angiotensin II/angiotensin type 1 receptor (ACE/angiotensin II/AT1R) axis
-
2.
Axis angiotensin-converting enzyme 2/Mas receptors (ACE2/MasR)
-
3.
Axis angiotensin-converting enzyme 2/Des-Arg9-bradykinin/bradykinin B1 (ACE2 /DABK/ bradykinin B1)
-
4.
Axis of complement factors 3a, 5a, and their receptors (C3a-C3aR/C5a-C5aR) [18]
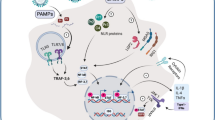
SARS-CoV-2 penetration into target lung cells occurs through binding of viral surface S-proteins with the cellular receptor for angiotensin-converting enzyme 2 (ACE2) [19]. Attachment to ACE2 receptor triggers an internalization of the complex into the target cell, leading to the subsequent suppression of ACE2 [20]. This further leads to the unimpeded function of angiotensin II (AngII) and a decrease in the level of angiotensin-(1–7) [21]. Because angiotensin-(1–7) plays a key counterregulatory role in many angiotensin type 1 receptor (AT1R)–related physiopathological functions, SARS-CoV-2-mediated downregulation of ACE2 and, as a result, increased total Ang II to angiotensin-(1–7) result in poor lung function and damage.
An imbalance of ACE2/ACE levels during COVID-19 infection and the dysregulation of the angiotensin II/AT1R axis of the renin–angiotensin–aldosterone system (RAAS) (a signaling pathway responsible for the regulation of blood pressure) underlie the cytokine storm and the associated acute lung damage [22]. These events result in a decrease of angiotensin [20]; an increase in the secretion of TFG-β, IL-1, IL-10, IL-12, and TNFα cytokines [21]; and activation of a complement system, including C5a and C5b (a complex of proteolytic enzymes of humoral immunity) [23], which in turn activates a kinin-kallikrein system (a group of proteins involved in inflammation, coagulation, and blood pressure control) [23].
Thus, caused by a COVID-19 infection, an imbalance of ACE2/ACE levels, and dysregulation of the angiotensin-II/AT1R axis of a renin–angiotensin–aldosterone system (RAAS) underlie the cytokine storm and associated acute lung injury [21].
The abovementioned cascade of biochemical reactions in patients with COVID-19 results in an increase level of cytokines: IL-1, IL -2, IL -6–10, IL -12, IL -17, IL -18, CSF, GM-CSF, and 4TNF-α [24]. As mentioned earlier, it is IL-6 that is considered pivotal to the development of a cytokine storm and mediates many of the biological effects shown in Fig. 1.
The mechanism of IL-6 action is quite complex: it is known that the cytokine interaction between the membrane protein IL-6R and the co-receptor gp130 has tissue homeostatic and reparative responses [25]. However, many non-immune cells, including stromal and epithelial cells, can induce pronounced inflammatory responses when the soluble IL-6R-IL-6 complex anchors on the gp130 membrane in what is known as trans-signaling, thus activating inflammatory responses [26]. Experimental studies show that murine lung stromal cells, including myofibroblasts, signal through both IL-6R and trans-signaling, but type 2 pneumocytes lack the soluble membrane receptor IL-6, indicating that these signals are transmitted exclusively through IL-6R trans-signaling [27]. Considering that trans-signaling usually causes inflammatory responses, this fact proves the importance of IL-6 in the development of ARDS in COVID-19 and disease outcomes [28].
With regard to the mechanism of action of IL-1, the SARS-Cov-2 virus causes damage to the epithelium, leading to the release of IL-1α [29], which in turn causes neutrophils and monocytes to move to the site of infection and [30] induces the release of IL-1β in monocytes/macrophages. In addition, pro-IL-1β in monocytes/macrophages even strongly activates the IL-1 release, which will in turn activate more innate immune cells. This autoinflammatory loop, in which IL-1 (IL-1α and IL-1β) can induce the production and release of more IL-1, must be tightly regulated as the ongoing loop activates more innate immune cells regardless of the initial trigger. Accordingly, blocking the IL-1 receptor (IL-1R) prevents autoinflammation by blocking the effects of IL-1α released from dead epithelial cells, as well as IL-1β produced by immune cells [29, 30].
It is also important to say that the soluble TNF-α receptor plays a large role in the development of the cytokine storm. TNF-α receptor is produced primarily by monocytes/macrophages as a transmembrane precursor, and a number of other immune and structural cell types such as T and B lymphocytes, mast cells, neutrophils, fibroblasts, and airway epithelial cells secrete it as a precursor. All these cell types produce cytokines [31, 32]. The TNF-α precursor is split by TNF-α-converting enzyme (TACE) to release TNF-α, which acts by binding to two different membrane receptors on target cells: TNFR1 and TNFR2 [33].
TNFR1 is widely expressed in the lymphoid system and in almost every cell in the body, which probably explains the wide range of functions of TNF-α. TNFR2 expression is limited to certain lymphocyte populations, including regulatory T cells [34]. Generally, binding of TNF-α to TNFR1 results in apoptosis due to the presence of death domains, and binding to TNFR2 results in cell survival, although there is some degree of overlap depending on the cell’s activation state and other factors [35]. Circulating TNFR1 immune complexes have been shown to be associated with adverse outcomes and severity of COVID-19 infection [36].
Thus, the effects of IL-6, IL-1, and TNFα on the development of a cytokine storm can be summarized using the scheme in Fig. 2.
2.2 Clinical Manifestations
Despite the fact that etiological causes of a cytokine storm may differ, clinical manifestations are often similar [37]. The most common symptoms are fever, fatigue, anorexia, headache, rash, diarrhea, arthralgia, myalgia, and neuropsychiatric disorders [38]. Respiratory symptoms are also reported: cough and tachypnea, which can progress to acute respiratory distress syndrome (ARDS). Renal failure, acute liver injury, or cholestasis may develop in severe cases of cytokine storm [39]. Cytokine storm syndrome can also cause disseminated intravascular coagulation, multiple organ failure, which can eventually lead to death [13].
As for laboratory diagnostics, it is rather difficult to mark any pathognomonic markers that would definitely predict the development of a cytokine storm. To a certain extent, levels of non-specific markers of inflammation, such as C-reactive protein (CRP) increase, correlate with severity of the disease [40]. Many patients develop hypertriglyceridemia and various changes in parameters of a complete blood count, such as leukocytosis or leukopenia, anemia, and thrombocytopenia, and—in biochemical panel—an increase in the level of ferritin and D-dimer. There is also an increase of certain serum inflammatory cytokines, such as IFN-γ, IL-6, and IL-10 [38].
It is extremely important to predict cytokine storm and find the adequate treatment [41]. However, finding the correct treatment proved to be complicated. Theoretically, it can include three main steps: identifying the underlying disease that caused the development of cytokine storm; determining the severity of a patient’s condition; and finding an appropriate treatment [40]. Since cytokine hyperproduction is fundamental in the pathophysiology of cytokine storm, direct blockade of these molecules may be one of the possible ways for cytokine storm treatment. Drugs, based on targeted blocking of certain molecules, have been widely used for the treatment of cytokine storm, including COVID-19-associated cytokine storm [42].
3 The Possibility of Using Monoclonal Antibody Drugs for COVID-19
The main idea behind monoclonal antibody (mAB) drugs is their targeted effect on the specific molecules, achieved through theirs specificity to a certain antigen [43]. Targeted removal of specific molecules made it possible to use these drugs not only for autoimmune diseases, but also in the complex treatment of patients with severe COVID-19, by directly blocking the action of cytokines. However, it is also important to take into account the side effects and contraindications that these drugs have [15].
Despite the fact that mABs can be extremely effective in the treatment of many infectious diseases, including COVID-19, large-scale production of such drugs is rather expensive, requires a lot of resources, which makes production expensive and not widely spread [44]. It should also be noted that nowadays there are many ongoing clinical studies aimed to determine the pharmacokinetics and pharmacodynamics of new monoclonal antibodies that could be beneficial for patients with COVID-19 [45].
It is already known that interleukin (IL)-6, as well as IL-1β, tumor necrosis factor-α, and interferon-γ play the key roles in the cytokine storm development in patients with COVID-19 [2, 3]. Moreover, according to a study conducted at the Wuhan Hospital, China, led by Chen X et al., it was found that an increase in IL-6 over 80 pg/ml increased the risk of developing respiratory failure by 22 times [46]. Therefore, the use of monoclonal antibodies that block the main cytokine storm mediators should improve the course of a disease in patients with COVID-19. Previously, in a prospective observational study conducted by a team of scientists from Spain, the main effect of monoclonal antibodies was studied in patients with autoimmune diseases such as polymyalgia rheumatica (PMR), vasculitis, systemic sclerosis (SS), Sjogren’s syndrome (SjS), systemic lupus erythematosus (SLE), and chronic inflammatory arthritis (rheumatoid arthritis (RA) inflammatory polyarthritis, psoriatic arthritis (PA), and ankylosing spondyloarthritis (AS)). The authors concluded that there was a blockade of an IL-6-dependent pro-inflammatory cascade, and, as a result, there is a decrease in the level of IL-6 and acute phase proteins (C-reactive protein, serum protein amyloid A, hepcidin, fibrinogen, etc.) [47]. According to a study led by Santos et al., the use of monoclonal antibodies in patients with rheumatism was not associated with severe manifestations of COVID-19; moreover, IL-6 inhibitors could show a protective effect [48].
3.1 Indications for Use
Nowadays the US Food and Drug Administration (FDA) granted an emergency use approvals for five investigational mABs [49]: bamlanivimab-etesevimab, casirivimab-imdevimab, sotrovimab, tixagevimab-silgavimab, and bebtelovimab. Bamlanivimab-etesevimab, casirivimab-imdevimab, sotrovimab, and bebtelovimab are approved for treatment of patients with mild to moderate COVID-19 disease who are at a high risk of progression to severe COVID-19 and/or hospitalization [50]. Casirivimab-imdevimab and bamlanivimab-etesevimab can be used for patients who had been recently exposed to COVID-19, immunocompromised, or unvaccinated [51].
According to the latest Russian clinical guidelines dated February 22, 2022, there are certain specific criteria for the use of IL-6 receptor blockers in the COVID-19 treatment. An IL-6 blocker or an IL-1α/IL-1β receptor antagonist is typically prescribed when a patient has a moderate course of COVID-19 and/or having risk factors for a severe course in the presence of pathological changes in lungs, in combination with two or more abnormal parameters. These include blood oxygen saturation (SpO2), CRP, body temperature, leukocyte count, absolute lymphocyte count, serum ferritin level, and lactate dehydrogenase (LDH) level [52].
3.2 Therapy Efficiency
Although IL-6 inhibitors have not yet been officially registered in clinical guidelines for the treatment of COVID-19, they have been widely used in pilot studies. For example, levilimab (an IL-6 blocker) was assessed in critically ill patients with COVID-19 who did not require artificial lung ventilation [53]. There were also randomized controlled trials using IL-6 blockers versus standard treatment or placebo for people with COVID-19 regardless of disease severity [54]. There is a lot of information available on the effectiveness and safety of these drugs.
Thus, according to the results of a multicenter, randomized, double-blind, placebo-controlled phase III clinical trial evaluating IL-6 blockers, which included 217 patients in total, levilimab was effective in achieving clinical improvement in patients hospitalized with severe COVID-19 pneumonia. Of patients in levilimab and placebo groups, 63.1% and 42.7%, respectively, achieved stable clinical improvement on day 14 (P = 0.0017). The frequency of adverse reactions was comparable between the groups [53].
There is also a systematic review made on the basis of the World Health Organization’s international clinical trial registry platform and the Living Overview of the Evidence (L-OVE) platform, as well as the Cochrane COVID-19 research registry. A total of 10 randomized controlled trials (RCTs) were included in the review with a total of 6896 people who were treated with IL-6 blocking agents such as tocilizumab and sarilumab compared to the standard treatment alone or placebo, regardless of the disease severity. The authors concluded that tocilizumab reduced the mortality rate (from any cause) after 28 days; whereas, sarilumab, on the contrary, did not show a statistically significant effect because the data on the effect of sarilumab are uncertain since RCTs have low reliability [55].
In addition to IL-6 receptor blockers, IL-1 blockers are currently being used, which are also being studied at the moment [56]. Anakinra, a recombinant interleukin-1 receptor antagonist, is effective in reducing clinical signs of hyperinflammation in critically ill COVID-19 patients. However, additional studies are needed for a more accurate conclusion [57, 58]. It has also been studied that an early treatment with anakinra under the control of soluble plasminogen urokinase activator receptor (suPAR) reduced severe respiratory failure and restores pro- and anti-inflammatory balance [56].
IL-17 participates in the development of hyperinflammatory syndrome, and it may be a new target in the treatment of COVID-19. The efficacy and safety of the IL-17 inhibitor natakimab was demonstrated in a retrospective case–control study in patients with severe COVID-19 outside the intensive care unit. Study demonstrated that the therapy reduced and moderated the inflammatory response and improved oxygenation, but did not affect the need for mechanical ventilation or mortality rate [59].
3.3 Side Effects and Long-Term Effects
Despite the large evidence base for the effectiveness mABs, these drugs have a wide range of side effects [16, 52, 56].
Usually side effects are associated with a drug action mechanism. The first thing to consider in when prescribing mABs is the possibility of developing an allergic reaction during the first and repeated injection of the drug. More often, local reactions, such as itching, rash, edema, erythema, and pain, occur when administered subcutaneously [15].
Depletion of T and B lymphocyte pools is possible, which leads to a decrease in barrier functions and activation of opportunistic infections. This is typical for tocilizumab [56].
For blockers of interleukins, systemic complications were noted: an increase in blood pressure, thromboembolism, a decrease in the filtration capacity of kidneys, changes in the production of thyroid hormones, and an inadequate functioning of the nervous system and gastrointestinal tract. Moreover, studies indicated a risk of developing secondary autoimmune diseases such as psoriasis and demyelinating diseases [60]. Violation of lipid metabolism is also one of the side effects of the use of IL-6 blockers [52].
According to the latest guidelines of the Ministry of Health of the Russian Federation (February 2022) contraindications for using mAbs (IL-6 receptor, Janus kinase blockers, IL-1) are sepsis that did not develop due to COVID-19; hypersensitivity to the components of a drug; and presence of a viral hepatitis B. Contraindications also include the presence of a concomitant diseases associated with an unfavorable prognosis; use of immunosuppressive therapy in organ transplantation; an increase in the activity of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) by more than 5 times in contrast to the normal range; thrombocytopenia < 50 × 109 /l; neutropenia < 0.5 × 109 /l; [52].
3.4 Features of a New SARS-CoV-2 Variant and the Effectiveness of Monoclonal Antibodies Used
Adaptive mutations in the SARS-CoV-2 genome change its pathogenic potential. This can have a significant effect on the course of the pandemic as a whole. Several variants of SARS-CoV-2 have been described since the beginning of the pandemic [61]:
-
Alpha (B.1.1.7): first variant of concern reported in the United Kingdom (UK) at the end of December 2020.
-
Beta (B.1.351): first reported in South Africa in December 2020.
-
Gamma (P.1): first reported in Brazil early January 2021.
-
Delta (B.1.617.2): first reported in India in December 2020.
-
Omicron (B.1.1.529): first registered in South Africa in November 2021.
Omicron is known to have more than 30 changes in the spike protein [62]. New mutations resulted in an increase in viral infectivity by 13 times, making it 2.8 times more infectious than the Delta variant [63]. It also has a shorter incubation period—only 3 days. Additionally, because the virus replicates in the upper respiratory tract, it causes less lung damage than previous variants of concern [64].
Scientists believe that a higher transmission rate combined with a very low pathogenicity of the Omicron variant will contribute to the formation of a herd immunity, giving hope for the end of the pandemic [65]. Among the main symptoms exhibited by patients, contracted Omicron variant, there is no loss of taste or smell; most patients do not present severe symptoms and do not require hospitalization [66]. According to the ICMR study, people infected with Omicron have a strong immune response capable of neutralizing not only Omicron but also other SARS-CoV-2 variants of concern. This may help to reduce the risk of reinfection with the Delta variant [67].
Previously approved monoclonal antibodies showed a reduced efficacy against the Omicron variant and their emergency use authorization was subsequently withdrawn. Sotrovimab is currently the only approved monoclonal antibody as it remains effective against this SARS-CoV-2 variant of concern [51]. Currently, only bebtelovimab remains effective against the Omicron BA.2 SARS-CoV-2 variant, so it is the only monoclonal antibody used to treat patients, infected with the new strain. Nevertheless, according to the FDA, the Omicron variant of SARS-CoV-2 is not treatable with the drugs casirivimab-imdevimab and bamlanivimab-etesevimab; therefore, it cannot be used as an effective therapy [49].
3.5 Efficacy and Safety of IL-6 Receptor Inhibitors in COVID-19 Patients With or Without Autoimmune Diseases
Nowadays there is a lot of information available about the correlation of elevated levels of pro-inflammatory cytokines such as IL-6, IL-1β, TNF-α, interferon-γ, macrophage inflammatory proteins 1α and 1β, and other cytokines with an acute, life-threatening respiratory injury, observed in patients with COVID-19 [2, 3]. Among these cytokines, interleukin (IL)-6 appears to play the key role in the pathogenesis of acute respiratory distress syndrome associated with COVID-19. The results of a meta-analysis showed that 53% of patients with COVID-19 had an elevated concentration of IL-6 [68]. A meta-analysis of 23 clinical trials involving 3400 patients showed that patients with severe COVID-19 had higher concentrations of IL-6 compared to the patients with a mild form. Even higher concentrations were observed in patients who died from COVID-19 complications [69]. Two additional meta-analyses [70, 71] and a large prospective cohort study of patients admitted to hospital with COVID-19 [72] also found an association between elevated IL-6 levels and COVID-19-related mortality rate. Many of these results were published during the first months of the pandemic. Since it was the elevated level of IL-6 that attracted attention among other cytokines, the majority of studies are focused on IL-6 and monoclonal antibodies that block this cytokine [73, 74]. Since Omicron to a lesser extent causes the development of respiratory distress syndrome, it does not lead to the development of severe stages of COVID-19, therefore monoclonal antibodies are almost not used for the treatment of the Omicron variant of SARS-CoV-2. Moreover, Omicron is not neutralized by monoclonal antibodies so they are not currently recommended [49].
Drugs that block IL-6 include levilimab, tocilizumab, and sarilumab or olokizumab, a monoclonal antibody belonging to the immunoglobulin G4/kappa isotype (it binds more selectively to human IL-6) [52]. The use of tocilizumab in the USA began in 2017 for a treatment of cytokine release syndrome (CRS) and macrophage activation syndrome (MAS) that developed in patients with autoimmune diseases. The first data on its effectiveness in the treatment of COVID-19 appeared in March 2020. A Russian innovative biotechnology company BIOCAD registered the IL-6 receptor inhibitor levilimab under the brand name Ilsira in 2020 [72].
Up to date, the use of IL-6 receptor inhibitors was mainly studied in patients with autoimmune diseases. For example, tocilizumab is approved by the European Alliance of Associations of Rheumatologists for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, giant cell arteritis, cytokine release syndrome, and idiopathic multicentric Castleman’s disease (iMCD) [75, 76], while siltuximab was only approved for iMCD [77] and sarilumab for rheumatoid arthritis [74].
Nevertheless, there is a number of studies [53, 74, 78, 79] confirming the effectiveness of IL-6 receptor inhibitors not only as a treatment in patients with impaired functioning of the immune system, but also for COVID-19.
A double-blind, placebo-controlled phase III clinical trial of the use of levilimab in patients with severe COVID-19 was conducted in the Russian Federation [53]. A total of 206 patients hospitalized for severe COVID-19 pneumonia were included in the study: 103 patients received levilimab and 103 patients received placebo. The proportion of patients who achieved sustained clinical improvement at day 14 and did not require rescue therapy was significantly higher in the levilimab group than in the placebo group (63.1% (65/103) versus 42.7% (44/ 103); P = 0.0017). The difference in the rate of sustained clinical improvement between the levilimab and placebo groups was 20.4%, with a one-sided 97.5% CI (7–100) (P = 0.0017); its lower limit was above the established superiority limit. Thus, the hypothesis of the efficacy of levilimab over placebo was confirmed [53].
There is an international clinical study, led by Chamlagain et al., that studied the use of sarilumab in patients admitted to the hospital with severe or extremely severe COVID-19 [74]. A randomized, double-blind, placebo-controlled, multinational study was conducted over 60 days at 45 hospitals located in Argentina, Brazil, Canada, Chile, France, Germany, Israel, Italy, Japan, Russia, and Spain. Patients were randomized (2:2:1) to a single dose of intravenous sarilumab 400 mg, sarilumab 200 mg, and placebo. On day 29, there was no significant difference in patients’ improvement between placebo (12 × 0 days [95% CI 9 × 0 to 15 × 0]) and sarilumab 200 mg (10 × 0 days [9 × 0 to 12 0] and higher dose of sarilumab 400 mg (10 0 days [9 0–13 0]; RR 1 14 [95% CI 0 84—1 54]; log-rank p = 0 34). There was no difference in survival rate (77 [92%] of 84 patients in the placebo group; 143 [90%] of 159 patients in the sarilumab 200 mg group; difference -1 7 [-9 3–5 8]; p = 0 63 versus placebo; and 159 [92%] of 173 patients in the sarilumab 400 mg arm; difference 0 2 [-6 9–7 4]; p = 0 × 85 versus placebo.) On day 29, there were non-significant differences in survival rate between sarilumab 400 mg (88%) and placebo (79%; + 8 × 9% difference [95% CI -7 7–25 5]; p = 0 25) in patients with critical illness. The incidence of treatment-related adverse events was 65% (55 of 84) in the placebo group, 65% (103 of 159) in the sarilumab group 200 mg, and 70% (121 of 173) in the sarilumab 400 mg group. Despite small clinical improvements in patients, the authors reported that day 29 survival was 9% higher in the sarilumab group than in the placebo group, specifically in patients who required non-invasive or invasive mechanical ventilation or extracorporeal membrane oxygenation. Thus, the results of this study did not exclude a possible benefit of sarilumab in patients admitted to the hospital with COVID-19 pneumonia [74].
One of the IL-6 receptor blockers that may be used for COVID-19 treatment is tocilizumab. It was introduced in the early 2000s for the treatment of autoimmune diseases such as refractory rheumatoid arthritis and systemic juvenile idiopathic arthritis (SJIA) [80, 81] and since 2017 has been approved by the FDA for the treatment of cytokine release syndrome (CRS), which can occur after certain forms of CAR-T cell-induced immunotherapy.
With regard to tocilizumab, several clinical guidelines, such as the Chinese guideline written by the National Health Commission and Public Administration [82] and the Italian Society for Infectious and Tropical Diseases (SIMIT) [83], already include tocilizumab as a therapeutic option for COVID-19. In a systematic review led by A. Cortegiani et al., 3 indirect preclinical studies and 28 clinical studies were identified, eventually including 5776 patients with COVID-19 using tocilizumab as treatment, but no significant effect on improving the course of the disease was found [84]. However, recent Chinese guidelines [82] suggested using tocilizumab 4–8 mg/kg when treating patients with extensive lung disease and severe conditions with elevated IL-6 or complications. The Italian Society for Infectious and Tropical Diseases does not reject the use of tocilizumab in a COVID-19 treatment [83] but recommends to select patients who have an initial high viral load, or patients with high IL-6 levels (> 40 pg/mL), patients with elevated D-dimer, C-reactive protein, ferritin, or fibrinogen levels and those requiring mechanical ventilation [83]. However, the National Institutes of Health (NIH) argued that the evidence was insufficient to make recommendations for the use of tocilizumab in COVID-19 [78]. The World Health Organization (WHO) and the Infectious Diseases Society of America (IDSA) recommend the use of tocilizumab only in the context of clinical trials [84, 85].
A team of researchers from Russia [79] analyzed the use of olokizumab in patients with COVID-19. They studied the efficacy and safety of Artlegia (olokizumab) in real clinical practice. The study included 610 patients aged 55.08 ± 12.68 years who received only 1 dose of olokizumab 160 mg/ml—0.4 ml subcutaneously as an anti-inflammatory therapy. The comparison group consisted of 511 patients aged 55.23 ± 11.23 years who received standard therapy without the use of IL-6 inhibitors. As a result, the use of olokizumab in COVID-19 demonstrated a positive effect on clinical and laboratory parameters. There was a registered general condition improvement on the first day of observation: a decrease in body temperature to normal values. Also, the marker of the acute phase of inflammation (C-reactive protein) decreased after treatment with IL-6 blockers, which indicates their positive effect [79].
When comparing different IL-6 receptor inhibitors used in the COVID-19 treatment, it must be said that they have different molecular structure. For example, tocilizumab and olokizumab were obtained in a humanized way, but levilimab has the greatest affinity for humans, and sarilumab has the least affinity for humans [54]. The mechanism of action of levilimab, tocilizumab, and sarilumab is based on the blockade of soluble and membrane IL-6 receptors, whereas olokizumab inhibits IL-6 directly, resulting in the suppression of the IL-6-dependent pro-inflammatory cascade. Moreover, there is a specific feature of levilimab which is a rapid increase in blood concentration (therapeutic values are reached by the 2nd day, the maximum by the 4th day), and the blockade of 90% of IL-6 membrane receptors occurs already in the first hours after the injection of this drug [54].
The COVID-19 pandemic has had a significant impact on people with rheumatoid arthritis (RA). Many of them are being treated with synthetic disease-modifying antirheumatic drugs (DMARDs) [86]. Although DMARDs are important in controlling autoimmune disease activity, their impact on COVID-19 outcomes in people with RA remains unclear. This uncertainty led to unnecessary anxiety, social isolation, and discontinuation of DMARD treatment, which may result in RA outbreaks [87,88,89]. The impact of DMARDs on COVID-19 outcomes is of particular interest because some of these drugs, such as tocilizumab and baricitinib, have been studied as repurposed treatment for COVID-19. For example, treatment with interleukin-6 (IL-6) inhibitors and baricitinib improved outcomes in some clinical trials among patients with COVID-19 [90,91,92,93].
According to a study led by the Rheumatological Alliance Global Registry of Physicians [94], patients who took IL-6 blockers as a treatment for autoimmune diseases had significantly better disease outcome than patients on other immunosuppressive drugs. A total of 2869 people with rheumatoid arthritis were analyzed (mean age 56.7 years, 80.8% women). Prior to the diagnosis of COVID-19, there were 237 people taking abatacept, 364 rituximab, 317 IL-6 inhibitors, 563 -anus kinase inhibitors, and 1388 tumor necrosis factor inhibitors [95]. People with rheumatoid arthritis who used rituximab or Janus kinase (JAK) inhibitors early in their COVID-19 disease were more likely to experience poor COVID-19 outcomes, ranging from hospitalization to death, compared to those using tumor necrosis factor inhibitors. In the end, 80 patients (22.0%) among those using rituximab required hospitalization with oxygen or ventilation support, and 54 (14.8%) died [95,96,97]. One hundred three patients (7.4%) who were taking tumor necrosis factor inhibitors required hospitalization and 36 people died (2.6%). Among JAK inhibitor users, 86 patients (15.3%) who needed oxygen/ventilation were hospitalized and 40 patients (7.1%) died. Only 9 (2.8%) patients who used IL-6 blockers died, which proves their best efficacy [94,95,96,97].
3.6 Use of IL-1 Receptor Inhibitors in COVID-19 Patients
The pro-inflammatory cytokine interleukin IL-1β is well known to play an important role in the development of macrophage activation syndrome (MAS), as it triggers cytokine production, including IL-1β itself, activates endothelial activation with fluid extravasation, and can lead to hypotension and even death [98]. Macrophage activation syndrome is an acute episode of overwhelming inflammation characterized by the activation and expansion of T lymphocytes and hemophagocytic macrophages. In rheumatology, it most commonly occurs in patients with systemic juvenile idiopathic arthritis (SJIA) and systemic lupus erythematosus [99]. However, there were cases of an increased IL-1 in patients with COVID-19 [100]. The group of mediators that control inflammatory responses to tissue damage during COVID-19 includes IL-1α and IL-1β: IL-1α is released by dying epithelial and endothelial cells, while IL-1β is produced by infiltrating monocytes, macrophages, and neutrophils [101]. The main endogenous regulatory mechanism preventing excessive IL-1-mediated inflammation is an IL-1 receptor antagonist [102]. In an ARDS study evaluating bronchoalveolar lavage cytokines in patients with COVID-19, it was shown that IL-1β concentrations reached peak levels at the onset of the disease; however, the physiological mechanism for dampening the excessive inflammatory response in the lung in COVID-19 was ultimately carried out by the antagonist IL-1 receptor [103]. Currently, there are several drugs blocking IL-1, but the most studied and widely used drug is anakinra [49].
Anakinra, a recombinant IL-1 receptor antagonist (IL-1RA), is currently used in the treatment of patients with RA, cryopyrin-associated periodic syndrome, and Still’s disease. It was shown to be effective in treating a subset of severe patients with bacterial sepsis associated to macrophage activation syndrome [102]. However, the FDA and the European Medicines Agency have already approved anakinra, in the treatment of COVID-19, but only in the context of a clinical study [49]. It is widely available and may represent a safe and controlled way to reduce inflammation in patients with COVID-19 [104]. But with the Omicron variant of SARS-CoV-2, there is no data confirming the use of IL-1 blockers as a treatment due to its resistance to monoclonal antibodies [49].
Moreover, there is a number of studies [105,106,107] confirming that anakinra may be effective in the treatment of COVID-19 patients that show signs of macrophage activation syndrome, especially those with persistent high fever and elevated plasma ferritin levels [108].
In December 2021, the European Medicines Agency approved the use of anakinra for the treatment of COVID-19 in adults with pneumonia who required supplemental oxygen (low or high flow oxygen) and were at risk of developing severe respiratory failure, determined by blood levels of a protein called suPAR (soluble urokinase plasminogen activator receptor) at least 6 ng per ml [105].
In a study based in Italian hospital, researchers assessed the use of anakinra in seriously ill patients with confirmed COVID-19. The total number of patients was 594 people: the experimental group received 100 mg per day of anakinra as an addition to the treatment for 10 days and the control group received 100 mg of placebo drug with the standard therapy. As a result, it was shown that an early administration of anakinra resulted in an improvement in overall clinical status in moderate and severe COVID-19 patients by 2.78 times and a decrease in 28-day mortality. At the same time, the medians of the main laboratory parameters of macrophage activation syndrome were as follows: CRP, mean (SD)-mg/l, 50.6 (25.3–99.7); IL-6, mean (SD)-pg/mL, 16.8 (7.0–39.8); and ferritin, mean (SD)-ng/ml, 585.2 (294.5–1.047.0) [105].
There is a study [106] that evaluated the efficacy of anakinra combined with methylprednisolone (a synthetic glucocorticoid drug) compared with the standard therapy (hydroxychloroquine, an antimalarial drug, azithromycin, a semi-synthetic antibiotic, low molecular weight heparin, an anticoagulant, and corticosteroids) in COVID-19 patients. The total number of patients was 120 with hyperinflammation syndrome and laboratory parameters: ferritin ≥ 1000 ng/ml and/or C-reactive protein > 10 mg/dl, including respiratory failure due to COVID-19. In this case, the dose of intravenous administration of anakinra was 200 mg for 3 days, and then 100 mg until the 14th day. After 28 days, the mortality rate was 13.9% in patients who received anakinra and methylprednisolone as a treatment and 35.6% in the control group. However, there was no significant difference in laboratory parameters [106].
Moreover, there is a study [107] in which patients with COVID-19-associated acute respiratory distress syndrome (ARDS), treated with anakinra, were retrospectively evaluated and compared with a cohort of control patients who did not receive immunomodulatory drugs. The primary endpoint was survival at day 28. The population consisted of 112 patients (56 received anakinra and 56 were in the control group). Survival on the 28th day in the experimental group was 61.6% and was significantly higher in patients treated with anakinra than in the control group (48.2%). Univariate analysis identified the use of anakinra (odds ratio 3.2; 95% confidence interval 1.47–7.17) as a significant predictor of survival. The frequency of adverse events associated with infectious complications was similar between the groups. Thus, anakinra improved overall survival rate, invasive ventilation-free survival rate, and was well tolerated by patients with ARDS in COVID-19 [107].
Since patients with autoimmune disorders infected with the SARS-CoV-2 have a high probability of a severe course of the disease and adverse outcomes, they need to be vaccinated [109]. In addition, there is data on the use of IL-1 receptor blockers during COVID-19 vaccination of patients with autoimmune diseases [107, 110, 111]. According to these studies, the authors highlighted three observations supporting the need for IL-1 blockade in patients with autoimmune rheumatic diseases during COVID-19 vaccination: (1) the presence of very high levels of IL-1 caused by the disease activity itself; (2) an additional increase in IL-1 in patients with COVID-19-associated cytokine storm; (3) better survival rates in patients with macrophage activation syndrome when treated with anakinra [102, 110, 111]. Similarly, data taken from a registry of children with multisystem inflammatory syndrome indicate that neither the underlying autoinflammatory disease no ongoing treatment with IL-1 blockers increase the risk of SARS-CoV-2 infection [109].
Anakinra, like other IL-1 blocking drugs, is used to treat COVID-19 because the rise in IL-1 levels precedes the increase in IL-6 levels in mice models of cytokine release syndrome, so according to these studies treatment with anakinra resulted in a decrease of both cytokines [112,113,114]. Anakinra has several additional advantages: (1) the short half-life time of anakinra allows the drug to be quickly withdrawn from the circulation [49]; (2) opportunistic infections in patients treated with anakinra are rare [115, 116].
3.7 Efficacy and Safety of the of the IL-17 Receptor Inhibitors Used in COVID-19 Patients With or Without Autoimmune Diseases
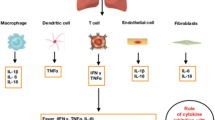
It is known that an increase of IL-17 stimulates the T cell response and in turn increases the production of other inflammatory mediators: IL-1β, IL-6, TNF-α, growth factors (G-CSF, GM-CSF). and various chemokines that play an important role in development of ARDS and MAS in COVID-19 patients [77]. IL-17A (commonly known as IL-17) is the best studied member of the IL-17 family of cytokines. It is secreted by T helper (Th)-17 lymphocytes [117]. In a recently published study by Parackova et al., Th-17 pathway activation was shown in patients with COVID-19 [45, 118]. Activation of the Th-17 pathway and production of IL-17 increased the reproduction of neutrophils and prevented their apoptosis, which ultimately increased the damage of the lung parenchyma and contributed to the development of pulmonary edema [77, 119]. In addition, it was suggested that IL-17 was involved in the development of endothelial dysfunction and thrombophilia in COVID-19 patients [120].
In addition, according to the methodological recommendations of the Russian Ministry of Health, netakimab, an IL-17 inhibitor, can be used in the treatment of non-severe COVID-19 patients [52]. A retrospective case–control study was conducted at the Sechenov Moscow Medical University and included 171 hospitalized patients with severe COVID-19. All patients were divided into 2 groups: experimental and control. The control group was comparable with the experimental group in terms of age, C-reactive protein level, oxygenation index ratio (SpO2/FiO), and the NEWS2 (National Early Warning Score) score. Patients in both study groups received standard therapy in the form of hydroxychloroquine (an antimalarial drug), azithromycin (a semi-synthetic antibiotic), low molecular weight heparin (an anticoagulant), and corticosteroids. In the experimental group, in addition to the standard therapy, patients also received netakimab (Efleira®, Biocad). In this study, administration of anti-IL-17 therapy to patients with severe COVID-19 was associated with improved clinical and laboratory parameters, although no significant effect on clinical outcomes, including mortality, was observed [121]. But with the Omicron variant of SARS-CoV-2, there is no data confirming the use of IL-17 blockers as a treatment due to its resistance to monoclonal antibodies [49].
There is not so much information about the outcome of COVID-19 in patients with autoimmune diseases receiving systemic therapy with IL-17 blockers [122, 123]. However, there is a retrospective, multicenter, observational study from northern Italy that included patients with chronic plaque psoriasis (n = 5206) who received a regular anti-IL-17 therapy and did not develop severe COVID-19 symptoms or died, which indirectly indicates a possible therapeutic effect of IL-17 blockers in the treatment of patients with COVID-19 [124].
3.8 Efficacy and Safety of IL-23 Receptor Inhibitors in COVID-19 Patients with Autoimmune Diseases
Similar to IL-17, IL-23 is an important cytokine in the maintenance of T helper cells that mediate defense mechanisms against pathogens. Although it is not thought to be a central cytokine in defense against viral infections, decreased levels of IL-23 may contribute to impaired mucosal barrier immunity, leading to an increased risk of respiratory infections [123]. Studies showed that Th17 may be involved in severe immune damage in COVID-19 patients [124, 125]. IL-23 inhibitors, through their action on Th17, may play an important role in attenuating key cytokines, possibly leading to a milder presentation of COVID-19 [119]. Currently, drugs specifically targeting IL-23 in the treatment of COVID-19 have been studied only in isolated cases in the treatment of people with psoriasis, who then suffered a coronavirus infection and had a more favorable course of COVID-19 disease [119, 126]. Therefore, there is no reliable data on the treatment of people with the Omicron variant of SARS-CoV-2 with IL-23 blockers.
A case of a COVID-19 patient with psoriasis who received an IL-23 inhibitor in the form of two Guselkumab injections was published. Within 2 months of the confirmed COVID-19, the patient was successfully cured without any complications. The authors concluded that inhibition of IL-23 did not have side effects under conditions of COVID-19 infection [119].
There is also a case of a 37-year-old man with a history of psoriasis who was hospitalized to the emergency department with a positive SARS-CoV-2 PCR test. He received risankizumab treatment for 4 months. The patient was discharged after 1 week without any complications, and after 2 weeks he fully recovered. According to the recommended dosing schedule for risankizumab (every 12 weeks after the initial loading dose at weeks 0 and 4), the patient received his next dose in April and experienced no adverse effects thereafter [126].
3.9 Efficacy and Safety of TNF-α Inhibitors in COVID-19 Patients With or Without Autoimmune Diseases
An animal study led by Karki R et al. showed that only the combination of TNF-α and IFN-γ induced inflammatory cell death characterized by panoptosis. Panoptosis is defined as an innate immune inflammatory programmed cell death pathway dependent on panoptosomes [127]. Panoptosomes are caspase-containing complexes [128]. TNF-α and IFN-γ caused a lethal effect due to the development of a cytokine storm in mice that reflected tissue damage and inflammation in COVID-19. Treatment with neutralizing antibodies against TNF-α and IFN-γ protected mice from mortality during SARS-CoV-2 infection, sepsis, hemophagocytic lymphohistiocytosis (an excessive activation of cytotoxic lymphocytes leading to functional tissue failure), and cytokine storm. The authors found that in a mice model of SARS-CoV-2, administration of a combination of anti-TNF and IFN-γ neutralizing antibodies increased survival from ≈14% to 50%. The authors concluded that blockade of these cytokines together or separately could prevent development of a severe COVID-19 [129]. Since TNF-α inhibitors are only being studied and are not included in the FDA clinical recommendations, their use in the omicron variant is also not reliably confirmed.
There are large studies databases on the use of TNF-α inhibitors in patients with COVID-19: there are 3 large registries, such as the Global Rheumatology Alliance (GRA), PsoProtect, and the Inflammatory Bowel Disease Registry (SECURE-IBD), which collected data on patients diagnosed with COVID-19 and immune-mediated inflammatory diseases treated with TNF-α inhibitors [130,131,132].
A registry called the Global Rheumatology Alliance (GRA) collected information from clinicians about patients with RA, diagnosed with COVID-19. Patients with an underlying rheumatic disease, who were treated with TNF-α inhibitors had a significantly lower odds of COVID-19-related hospitalization compared with no therapy (adjusted odds ratio [OR] 0.40, 95% confidence interval [CI] 0.19–0.81) [130].
The PsoProtect registry included clinically reported cases of SARS-CoV-2 infection in people with psoriasis. Patients who refused treatment with TNF-α inhibitors had a significantly higher chance of hospitalization (RR 2.84, 95% CI 1.31–6.18), reflecting the results of the GRA registry [131].
In the Inflammatory Bowel Disease Registry (SECURE-IBD) [133], there was no significant association with the use of TNF-α inhibitors (compared to no use) for the primary outcome of severe COVID-19, defined as the pool of admission to the intensive care unit, ventilator use and/or death (adjusted OR 0.9, 95% CI 0.4–2.2). However, drug use was inversely associated with a secondary outcome of hospitalization or death (adjusted RR 0.60, 95% CI 0.38–0.96), consistent with the other two registries [133, 134].
According to the study led by Stallmach et al., which retrospectively studied seven patients with severe COVID-19 but without any underlying immune-mediated inflammatory disease, patients received a single dose of infliximab 5 mg/kg 0 to 3 days after hospitalization. As a result, a drop in the level of pro-inflammatory cytokines was observed during the first 10 days after taking infliximab. And only 1 patient out of 7 died, but the authors noted that he was from an older age group [135].
3.10 The Effect of Genetically Engineered Cytokine Storm Suppressing Drugs on Vaccination Against COVID-19
There are several COVID-19 vaccines approved by WHO for use (subject to the emergency use list) [49]. The humoral immune response caused by the vaccine, especially those associated with the production of neutralizing antibodies, are crucial for limiting infection and preventing reinfection. Therefore, their use is justified. But at the same time, the vaccine against SARS-Cov-2 can lead to cytokine storm syndrome in some vaccinated people. So, scientists from the USA [136] studied the level of interleukins in 33 monkeys and 200 mice that were vaccinated and found that these animals were able to fight the virus well, which led to the rapid removal of the virus from their lungs, with the exception of two monkeys and 9 mice. These two monkeys, together with mice, had cytokine storm syndrome in the lungs. This result is extremely important for human vaccination. This syndrome was acute in one of the monkeys and in 4 mice. Two animals showed elevated plasma levels of IL-6 compared to baseline levels [136].
The first goal of all alternative vaccines against SARS-CoV-2 is to strengthen the immune system as much as possible, with often two vaccinations to create a strong immunization against COVID-19. It was, however, reported to worsen the cytokine syndrome. Therefore, it may be useful to use interleukin blockers when vaccinating people; alternatively, it might be helpful to develop a “smart” vaccine against SARS-Cov-2, which can deliver only the necessary dose of the virus to different people so as not to cause a cytokine storm.
As for people with autoimmune diseases receiving anti-cytokine therapy who require mandatory vaccination against COVID-19, there are several opinions. The most commonly accepted opinion is that the effectiveness of the COVID-19 vaccine will be lower in patients receiving interleukin blockers; however, limited data show that patients with chronic rheumatic diseases under immunosuppression develop adequate levels of antibody responses. With patients’ consent, vaccination can be recommended even without interrupting anti-cytokine therapy, especially in people with continuing high disease activity in order to avoid its relapses [137, 138].
4 Conclusion
In conclusion, monoclonal antibodies against IL-6, IL-1, IL-17, IL-23, and TNF-α receptors may reduce mortality rate in people with COVID-19 and are a promising addition to COVID-19 treatment. Moreover, they do not have strong side effects and are not associated with a severe course of the disease.
IL-6 inhibitors are currently the most studied GEDs; they are also included in the standards of pathogenetic treatment of cytokine release syndrome in COVID-19 in many countries (USA, Italy, China, and Russia). Therapy that is carried out in advance and includes IL-6 inhibitors provides inhibition of systemic inflammation and contributes to the suppression of cytotoxic shock syndrome and is also aimed at reducing the risk of multiple organ failure and death. Currently, the only monoclonal antibody used to treat patients infected with the new strain of SARS-CoV-2 Omicron BA.2 is bebtelovimab; the other monoclonal bodies have not shown their effectiveness.
Less studied are monoclonal antibodies against IL-1, IL-17, IL-23, and TNF-α receptors. However, recent systematic reviews and meta-analyses do not describe the negative effects of using these drugs in patients with severe COVID-19 or in people with autoimmune diseases. There is still no consensus on the timing, dosage, and applicability of these drugs to the world population, which requires further study.
Also, based on the analyzed data regarding patients who have previously been treated with monoclonal antibodies due to autoimmune diseases, it can be concluded that no side effects or aggravation of the disease were observed, moreover, it is not recommended to stop taking these drugs, as they can become a warning cytokine storm therapy (Table 1).
References
Sayganov, S. A., Mazurov, V. I., Bakulin, I. G., et al. (2020). Current, effectiveness of therapy and outcomes of new coronavirus infection: Preliminary analysis. Journal of North-Western State Medical University named after I.I. Mechnikov, 12(2), 27–38. https://doi.org/10.17816/mechnikov34932
Zhang, Y., Li, J., Zhan, Y., et al. (2004). Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infection and Immunity, 72(8), 4410–4415. https://doi.org/10.1128/IAI.72.8.4410-4415.2004
Lau, S. K. P., Lau, C. C. Y., Chan, K. H., et al. (2013). Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: Implications for pathogenesis and treatment. Journal of General Virology, 94(Pt 12), 2679–2690. https://doi.org/10.1099/vir.0.055533-0
Tufan, A., AvanoğluGüler, A., & Matucci-Cerinic, M. (2020). COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci., 50(SI-1), 620–632. https://doi.org/10.3906/sag-2004-168
Wong, J. P., Viswanathan, S., Wang, M., Sun, L. Q., Clark, G. C., & D’Elia, R. V. (2017). Current and future developments in the treatment of virus-induced hypercytokinemia. Future Medicinal Chemistry, 9(2), 169–178. https://doi.org/10.4155/fmc-2016-0181
Kim, J. S., Lee, J. Y., Yang, J. W., Lee, K. H., Effenberger, M., Szpirt, W., Kronbichler, A., & Shin, J. I. L. (2021). Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics, 11(1), 316–329. https://doi.org/10.7150/thno.49713
Ferrara, J. L., Abhyankar, S., & Gilliland, D. G. (1993). Cytokine storm of graft-versus-host disease: A critical effector role for interleukin-1. Transplantation Proceedings, 25, 1216–1217.
Barry, S. M., Johnson, M. A., & Janossy, G. (2000). Cytopathology or immunopathology? The puzzle of cytomegalovirus pneumonitis revisited. Bone Marrow Transplantation, 26, 591–597. https://doi.org/10.1038/sj.bmt.1702562
Imashuku, S. (2002). Clinical features and treatment strategies of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Critical Reviews in Oncology Hematology, 44(3), 259–272. https://doi.org/10.1016/S1040-8428(02)00117-8
Bisno, A. L., Brito, M. O., & Collins, C. M. (2003). Molecular basis of group A streptococcal virulence. The Lancet Infectious Diseases, 3, 191–200. https://doi.org/10.1016/S1473-3099(03)00576-0
Huang, K. J., Su, I.-J., et al. (2005). An interferon-gamma-related cytokine storm in SARS patients. Journal of Medical Virology, 75(2), 185–194. https://doi.org/10.1002/jmv.20255
Guan, W. J., Ni, Z. Y., Hu, Y., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382(18), 1708–1720. https://doi.org/10.1056/NEJMoa2002032
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., Zhang, L., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet, 395(10223), 497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Fang, Y., Zhang, H., Xu, Y., Xie, J., Pang, P., & Ji, W. (2020). CT manifestations of two cases of 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology, 295(1), 208–209. https://doi.org/10.1148/radiol.2020200280
Chen, L., Liu, H. G., Liu, W., Liu, J., Liu, K., Shang, J., et al. (2020). Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jiehe He Huxi Zazhi, 43, E005. https://doi.org/10.3760/cma.j.issn.1001-0939.2020.0005
Liu, B., Li, M., Zhou, Z., Guan, X., & Xiang, Y. (2020). Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? Journal of Autoimmunity, 111, 102452. https://doi.org/10.1016/j.jaut.2020.102452
Zhou, F., Yu, T., Du, R., Fan, G., Liu, Y., Liu, Z., et al. (2020). Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan China: A retrospective cohort study. Lancet, 395, 1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3
Mahmudpour, M., Roozbeh, J., Keshavarz, M., Farrokhi, S., & Nabipour, I. (2020). COVID-19 cytokine storm: The anger of inflammation. Cytokine, 133, 155151. https://doi.org/10.1016/j.cyto.2020.155151
Lan, J., Ge, J., Yu, J., Shan, S., Zhou, H., Fan, S., Zhang, Q., Shi, X., Wang, Q., Zhang, L., & Wang, X. (2020). Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature, 581, 215–220. https://doi.org/10.1038/s41586-020-2180-5
Vaduganathan, M., Vardeny, O., Michel, T., McMurray, J. J. V., & Pfeffer, M. A. (2020). Solomon SD Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med., 382(17), 1653–1659. https://doi.org/10.1056/NEJMsr2005760
Sano, M., Fukuda, K., Kodama, H., Pan, J., Saito, M., Matsuzaki, J., Takahashi, T., Makino, S., Kato, T., & Ogawa, S. (2000). Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. Molecular Basis of Cell and Development Biology, 275(38), 29717–29723. https://doi.org/10.1074/jbc.M003128200
Henry, B.M., Vikse, J., Lippi, G. (2020). COVID-19 induced Renin-Aangiotensin System (RAS) imbalance may drive acute lung injury: the evidence and therapeutic options. British Medical Journal, 368:m406. https://doi.org/10.1136/bmj.m406
Dong, C., Davis, R. J., & Flavell, R. A. (2002). MAP kinases in the immune response Annu. Revue d’Immunologie, 20(1), 55–72. https://doi.org/10.1146/annurev.immunol.20.091301.131133
Tanaka, T., Narazaki, M., & Kishimoto, T. (2016). Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy, 8(8), 959–970. https://doi.org/10.2217/imt-2016-0020
Mihara, M., Hashizume, M., Yoshida, H., Suzuki, M., & Shiina, M. (2011). IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clinical Science, 122(4), 143–159. https://doi.org/10.1042/CS20110340
Jones, S.A., Richards, P.J., Scheller, J., Rose-John, S. (2005). IL-6 transsignaling: the in vivo consequences. Journal of Interferon & Cytokine Research 25(5):241–53. https://doi.org/10.1089/jir.2005.25.241
Le, T.-T.T., Karmouty-Quintana, H., Melicoff, E., Le, T.-T.T., Weng, T., Chen, N.-Y., et al. (2014). Blockade of IL-6 Trans Signaling attenuates pulmonary fibrosis. The Journal of Immunology, 193, 3755–3768. https://doi.org/10.4049/jimmunol.1302470
McGonagle, D., Sharif, K., O’Regan, A., & Bridgewood, C. (2020). The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmunity Reviews, 19(6), 102537. https://doi.org/10.1016/j.autrev.2020.102537
Russell, C. D., Millar, J. E., & Baillie, J. K. (2020). Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet, 395(10223), 473–475. https://doi.org/10.1016/S0140-6736(20)30317-2
Lu, X., Chen, T., Wang, Y., Wang, J., & Yan, F. (2020). Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Critical Care, 24, 241. https://doi.org/10.1186/s13054-020-02964-w
Grivennikov, S. I., Tumanov, A. V., Liepinsh, D. J., Kruglov, A. A., Marakusha, B. I., Shakhov, A. N., et al. (2005). Distinct and nonredundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: Protective and deleterious effects. Immunity, 22, 93–104. https://doi.org/10.1016/S1074-7613(04)00379-6
Tay, M. Z., Poh, C. M., Rénia, L., MacAry, P. A., & Ng, L. F. P. (2020). The Trinity of COVID-19: Immunity, inflammation and intervention. Nature Reviews Immunology, 20(6), 363–374. https://doi.org/10.1038/s41577-020-0311-8
Horiuchi, T., Harashima, S., Tsukamoto, H., & Shimoda, T. (2010). Transmembrane TNF-α: Structure, function and interaction with anti-TNF agents. Rheumatol (Oxford England), 49, 1215–1228. https://doi.org/10.1093/rheumatology/keq031
Ware, C. F., Crowe, P. D., Vanarsdale, T. L., Andrews, J. L., Grayson, M. H., Jerzy, R., et al. (1991). Tumor necrosis factor (TNF) receptor expression in T lymphocytes. Differential Regulation of the Type I TNF Receptor During Activation of Resting and Effector T Cells. The Journal of Immunology, 147, 4229–4238.
Pimentel-Muiños, F. X., & Seed, B. (1999). Regulated commitment of TNF receptor signaling: A molecular switch for death or activation. Immunity, 11, 783–793. https://doi.org/10.1016/S1074-7613(00)80152-1
Mortaz, E., Tabarsi, P., Jamaati, H., Roofchayee, N. D., et al. (2021). Increased serum levels of soluble TNF-α receptor is associated with ICU mortality in COVID-19 patients. Frontiers in Immunology, 12, 592727. https://doi.org/10.3389/fimmu.2021.592727
Lee, D. W., Santomasso, B. D., Locke, F. L., et al. (2019). ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biology of Blood and Marrow Transplantation, 25, 625–638. https://doi.org/10.1016/j.bbmt.2018.12.758
Grupp, S. A., Kalos, M., Barrett, D., et al. (2013). Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. New England Journal of Medicine, 368, 1509–1518. https://doi.org/10.1056/NEJMoa1215134
Templin, C., Ghadri, J. R., Diekmann, J., et al. (2015). Clinical features and outcomes of takotsubo (stress) cardiomyopathy. New England Journal of Medicine, 373, 929–938. https://doi.org/10.1056/NEJMoa1406761
Lee, D. W., Gardner, R., Porter, D. L., et al. (2014). Current concepts in the diagnosis and management of cytokine release syndrome. Blood, 124, 188–195. https://doi.org/10.1182/blood-2014-05-552729
Fajgenbaum, D. C., & June, C. H. (2020). Cytokine Storm. New England Journal of Medicine, 383(23), 2255–2273. https://doi.org/10.1056/NEJMra2026131
Hu, B., Huang, S., & Yin, L. (2020). The cytokine storm and COVID-19. Journal of Medical Virology, 93(1), 250–256. https://doi.org/10.1002/jmv.26232
Avdeeva, Zh. I., Alpatova, N. A., Volkova, R. A., & Lapteva, L. K. (2011). Medicinal Preparations based on genetically engineered monoclonal antibodies. BIOpreparations. Prevention, diagnosis, treatment, 2, 14–19.
Jahanshahlu, L., & Rezaei, N. (2020). Monoclonal antibody as a potential anti-COVID-19. Biomedicine & Pharmacotherapy, 129, 110337. https://doi.org/10.1016/j.biopha.2020.110337
Deb, P., Molla, M. A., & Saif-Ur-Rahman, K. M. (2021). An update to monoclonal antibody as therapeutic option against COVID-19. Biosafety and Health, 3(20), 87–91. https://doi.org/10.1016/j.bsheal.2021.02.001
Chen, X., Zhao, B., Qu, Y., et al. (2020). Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clinical Infectious Diseases, 71(8), 1937–1942. https://doi.org/10.1093/cid/ciaa449
Santos, C. S., Morales, C. M., Álvarez, E. D., Castro, C. Á., Robles, A. L., & Sandoval, T. P. (2020). Determinants of COVID-19 disease severity in patients with underlying rheumatic disease. Clinical Rheumatology, 39(9), 2789–2796. https://doi.org/10.1007/s10067-020-05301-2
Santos, C. S., Férnandez, X. C., Moriano Morales, C., et al. (2021). Biological agents for rheumatic diseases in the outbreak of COVID-19: Friend or foe? RMD Open, 7(1), e001439. https://doi.org/10.1136/rmdopen-2020-001439
COVIDprotocols v2.0. Brigham and Women’s Hospital/Partners In Health/UCSF Institute for Global Health Sciences, 2021. https://www.covidprotocols.org Accessed April 3, 2022
COVIDprotocols v2.0. Brigham and Women’s Hospital/Partners In Health/UCSF Institute for Global Health Sciences (2021) https://www.covidprotocols.org. Accessed April 2, 2022
Emergency Use Authorization (EUA) (2021) Fact sheet for healthcare providers emergency use authorization of sotrovimab (EUA). https://www.fda.gov/media/149534/download. Accessed 2 April 2022
Temporary Methodological Recommendations Prevention, Diagnosis And Treatment New Coronavirus Infections (Covid-19), version 15. 2021 https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/059/392/original/%D0%92%D0%9C%D0%A0_COVID-19_V15.pdf
Lomakin, N. V., Bakirov, B. A., Protsenko, D. N., Mazurov, V. I., et al. (2021). The efficacy and safety of levilimab in severely ill COVID-19 patients not requiring mechanical ventilation: Results of a multicenter randomized double-blind placebo-controlled phase III CORONA clinical study. Inflammation Research, 70(10–12), 1233–1246. https://doi.org/10.1007/s00011-021-01507-5
ILSIRA (levimab) (2020) Clinical trial of Ilsira® (Levilimab). https://ilsira.ru/clinical_researches. Accessed April 2, 2022
Ghosn, L., Chaimani, A., Evrenoglou, T., Davidson, M., Graña, C., Schmucker, C., et al. (2021). Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane database of systematic reviews, 3(3), CD013881. https://doi.org/10.1002/14651858.CD013881
Bejan-Angoulvant, T., & Alexandre, J. (2019). Mechanism of action and adverse effects of monoclonal antibodies. Medical Science (Paris), 35(12), 1114–1120. https://doi.org/10.1051/medsci/2019208
Khan, F. A., Stewart, I., Fabbri, L., Moss, S., Robinson, K., Smyth, A. R., & Jenkins, G. (2021). Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax, 76(9), 907–919. https://doi.org/10.1136/thoraxjnl-2020-215266
Kooistra, E. J., Waalders, N. J. B., Grondman, I., Janssen, N. A. F., de Nooijer, A. H., Netea, M. G., van de Veerdonk, F. L., Ewalds, E., van der Hoeven, J. G., Kox, M., & Pickkers, P. (2020). RCI-COVID-19 Study Group. Anakinra treatment in critically ill COVID-19 patients: a prospective cohort study. Crit Care, 24(1), 688. https://doi.org/10.1186/s13054-020-03364-w
Avdeev, S. N., Trushenko, N. V., Tsareva, N. A., Yaroshetskiy, A. I., Merzhoeva, Z. M., et al. (2021). Anti-IL-17 monoclonal antibodies in hospitalized patients with severe COVID-19: A pilot study. Cytokine, 146, 155627. https://doi.org/10.1016/j.cyto.2021.155627
Sudakova, O. A., Demidova, M. A., & Kudrashova, M. N. (2021). Therapeutic monoclonal antibodies: A review of the literature. Upper Volga Medical Journal, 20(3), 26–31.
Aleem A, Akbar Samad AB, Slenker AK (2022). Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19) [Updated 2022 Feb 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; https://www.ncbi.nlm.nih.gov/books/NBK570580/
Callaway, E. (2021). Heavily mutated Omicron variant puts scientists on alert. Nature. Dec;600(7887):21. https://doi.org/10.1038/d41586-021-03552-w
Chen, J., Wang, R., Gilby, N.B., Wei, G.W. (2022). Omicron (B.1.1.529): Infectivity, vaccine breakthrough, and antibody resistance. Preprint. ArXiv 24;62(2):412–422.https://doi.org/10.1021/acs.jcim.1c01451
Brandal Lin T, Emily MacDonald, Lamprini Veneti, Tine Ravlo, Heidi Lange, Umaer Naseer, Siri Feruglio, Karoline Bragstad, Olav Hungnes, Ødeskaug Liz E, Frode Hagen, Hanch-Hansen Kristian E, Andreas Lind, Viksmoen Watle Sara, Taxt Arne M, Mia Johansen, Line Vold, Preben Aavitsland, Karin Nygård, & Madslien Elisabeth H (2021). Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Euro Surveill, 26(50), pii=101147. https://doi.org/10.2807/1560-7917.ES.2021.26.50.2101147
Kozlov, M. (2022). Omicron’s feeble attack on the lungs could make it less dangerous. Nature 601:177. https://doi.org/10.1038/d41586-022-00007-8
Wolter, N., Jassat, W., Walaza, S, Welch, R., Moultrie, H., Groome, M., Amoako, D.G., Everatt, J., Bhiman, J.N., Scheepers, C., Tebeila, N. (2021). Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa. Lancet 399(10323):437–446. https://doi.org/10.1101/2021.12.21.21268116
https://economictimes.indiatimes.com/industry/healthcare/biotech/healthcare/immune-responseinducedbyomicroneffectivelyneutralisedeltavarianticmrstudy/articleshow/89136215.cms?from=mdr (2022), Accessed 12th Mar 2022
Zhang, Z. L., Hou, Y. L., Li, D. T., & Li, F. Z. (2020). Laboratory findings of COVID-19: A systematic review and meta-analysis. Scandinavian Journal of Clinical and Laboratory Investigation, 80, 441–447. https://doi.org/10.1080/00365513.2020.1768587
Zhu, J., Pang, J., & Ji, P. (2020). Elevated interleukin-6 is associated with severity of COVID-19: A meta-analysis. Journal of Medical Virology. https://doi.org/10.1002/jmv.26085
Tian, W., Jiang, W., & Yao, J. (2020). Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. Journal of Medical Virology, 92, 1875–1883. https://doi.org/10.1002/jmv.26050
Zeng, F., Huang, Y., & Guo, Y. (2020). Association of inflammatory markers with the severity of COVID-19: A meta-analysis. International Journal of Infectious Diseases, 96, 467–474. https://doi.org/10.1016/j.ijid.2020.05.055
Samigullina, R. R., Mazurov, V. I., & Trofimov, E. A. (2021). Characteristics of complex therapy of immuno-inflammatory rheumatic diseases in COVID-19 pandemic conditions. Russian Medical Inquiry, 5(5), 260–267. https://doi.org/10.1136/10.32364/2587-6821-2021-5-5-260-267
Cummings, M. J., Baldwin, M. R., & Abrams, D. (2020). Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet, 395, 1763–1770. https://doi.org/10.1016/S0140-6736(20)31189-2
Lescure FX, Honda H, Fowler RA, Lazar JS, Shi G, Wung P, Patel N, Hagino O, Sarilumab COVID-19 Global Study Group. (2021). Sarilumab in patients admitted to hospital with severe or critical COVID-19: A randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. Respiratory medicine, 9(5), 522–532. https://doi.org/10.1016/S2213-2600(21)00099-0
Smolen, J.S., Landewé, R., Bijlsma, J., et al. (2017). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Annals of the Rheumatic Diseases 76(6):960–977. https://doi.org/10.1136/annrheumdis-2016-210715
Singh, J. A., Saag, K. G., Bridges, S. L. J., et al. (2015). American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis & Rhematology, 68(1), 1–26. https://doi.org/10.1002/art.39480
Pacha, O., Sallman, M. A., & Evans, S. E. (2020). COVID-19: A case for inhibiting IL-17? Nature Reviews Immunology, 20(6), 345–346. https://doi.org/10.1038/s41577-020-0328-z
NIH (2020) Immune-Based Therapy | Coronavirus Disease COVID -19. https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/. Accessed April 2, 2022
Antonov, V. N., Ignatova, G. L., Pribytkova, O. V., Sleptsova, S. S., Strebkova, E. A., et al. (2020). Experience of using olokizumab in patients with COVID-19. Therapeutic Archive, 12, 148–154. https://doi.org/10.26442/00403660.2020.12.200522
De Benedetti, F., Brunner, H. I., Ruperto, N., Kenwright, A., Wright, S., & Calvo, I. (2012). Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. New England Journal of Medicine, 367, 2385–2395. https://doi.org/10.1056/NEJMoa1112802
Singh, J. A., Beg, S., & Lopez-Olivo, M. A. (2010). Tocilizumab for rheumatoid arthritis. The Journal of Rheumatology, 38(1), 10–20. https://doi.org/10.3899/jrheum.100717
National Health Commission & State Administration, Traditional Chinese Medicine (2020) Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia. https://www.chinalawtranslate.com/wp-content/uploads/2020/03/Who-translation.pdf. Accessed 3 April 2022
SIMIT (2020) Vademecum per la cura delle persone con malattia da COVID-19. https://www.simit.org/news/11-vademecum-per-la-cura-delle-persone-con-malattia-da-covid-19. Accessed April 2, 2022
Cortegiani, A., Ippolito, M., Greco, M., Granone, V., Protti, A., Gregoretti, C., Giarratano, A., Einav, S., & Cecconi, M. (2021). Rationale and evidence on the use of tocilizumab in COVID-19: A systematic review. Pulmonology, 27(1), 52–66. https://doi.org/10.1016/j.pulmoe.2020.07.003
WHO (2022). WHO prequalifies first monoclonal antibody - tocilizumab – to treat COVID-19. WHO. https://www.who.int/news/item/11-02-2022-who-prequalifies-first-monoclonal-antibody---tocilizumab-to-treat-covid-19. Accessed 11 February 2022
D’Silva, K. M., & Wallace, Z. S. (2021). COVID-19 and rheumatoid arthritis. Current Opinion in Rheumatology, 33, 255–261. https://doi.org/10.1097/BOR.0000000000000786
Antony, A., Connelly, K., De Silva, T., et al. (2020). Perspectives of patients with rheumatic diseases in the early phase of COVID-19. Arthritis Care and Research, 72, 1189–1195. https://doi.org/10.1002/acr.24347
Ma, M. H. Y., Tay, S. H., Cheung, P. P. M., et al. (2021). Attitudes and behaviors of patients with rheumatic diseases during the early stages of the COVID-19 outbreak. Journal of Rheumatology, 48, 35–39. https://doi.org/10.3899/jrheum.200646
George, M. D., Venkatachalam, S., Banerjee, S., et al. (2020). Concerns, healthcare use, and treatment interruptions in patients with common autoimmune rheumatic diseases during the COVID-19 pandemic. Journal of Rheumatology, 48(4), 603–607. https://doi.org/10.3899/jrheum.201017
Gordon, A. C., Mouncey, P. R., et al. (2021). Interleukin-6 receptor antagonists in critically ill patients with Covid-19. New England Journal of Medicine, 384, 1491–1502. https://doi.org/10.1056/NEJMoa2100433
Salama, C., Han, J., Yau, L., et al. (2021). Tocilizumab in patients hospitalized with Covid-19 pneumonia. New England Journal of Medicine, 384, 20–30. https://doi.org/10.1056/NEJMoa2030340
Gupta, S., Wang, W., Hayek, S. S., et al. (2021). Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Internal Medicine, 181, 41–51. https://doi.org/10.1001/jamainternmed.2020.6252
Kalil, A. C., Patterson, T. F., Mehta, A. K., et al. (2021). Baricitinib plus Remdesivir for hospitalized adults with Covid-19. New England Journal of Medicine, 384, 795–807. https://doi.org/10.1056/NEJMoa2031994
Sparks, J.A., Wallace, Z.S., Seet, A.M., Gianfrancesco, M.A., Izadi, Z., Hyrich, K.L. et al. (2021). COVID-19 global rheumatology alliance associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: Results from the COVID-19 global rheumatology alliance physician registry. Annals of the Rheumatic Diseases 80(9):1137–1146. https://doi.org/10.1136/annrheumdis-2021-220418
Robinson, P. C., & Yazdany, J. (2020). The COVID-19 global rheumatology alliance: Collecting data in a pandemic. Nature Reviews Rheumatology, 16, 293–294. https://doi.org/10.1038/s41584-020-0418-0
Wallace, Z. S., Bhana, S., Hausmann, J. S., et al. (2020). The rheumatology community responds to the COVID-19 pandemic: The establishment of the COVID-19 global rheumatology alliance. Rheumatology, 6, 1204–1206. https://doi.org/10.1093/rheumatology/keaa191
Gianfrancesco, M., Hyrich, K. L., Gossec, L., et al. (2020). Rheumatic disease and COVID-19: Initial data from the COVID-19 global rheumatology alliance provider registry. Lancet Rheumatology, 2(5), 250–253. https://doi.org/10.1016/S2665-9913(20)30095-3
Schulert, G. S., & Grom, A. A. (2015). Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annual Review of Medicine, 66, 145–159. https://doi.org/10.1146/annurev-med-061813-012806
Hadchouel, M., Prieur, A. M., & Griscelli, C. (1985). Acute hemorrhagic, hepatic, and neurologic manifestations in juvenile rheumatoid arthritis: Possible relationship to drugs or infection. Journal of Pediatrics, 106, 561–566. https://doi.org/10.1016/S0022-3476(85)80072-X
Mehta, P., McAuley, D. F., & Brown, M. (2020). COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet, 395, 1033–1034. https://doi.org/10.1016/S0140-6736(20)30628-0
Dinarello, C. A. (2011). Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood, 117, 3720–3732. https://doi.org/10.1182/blood-2010-07-273417
Shakoory, B., Carcillo, J. A., Chatham, W. W., Amdur, R. L., Zhao, H., Dinarello, C. A., et al. (2016). Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: Reanalysis of a prior phase III trial. Critical Care Medicine, 44(2), 275–281. https://doi.org/10.1097/CCM.0000000000001402
Park, W. Y., Goodman, R. B., & Steinberg, K. P. (2001). Cytokine balance in the lungs of patients with acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine, 164, 1896–1903. https://doi.org/10.1164/ajrccm.164.10.2104013
Cavalli, G., De Luca, G., Campochiaro, C., Della-Torre, E., Ripa, M., Canetti, D., et al. (2020). Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol., 2(6), 325–331. https://doi.org/10.1016/S2665-9913(20)30127-2
Kyriazopoulou, E., Panagopoulos, P., Metallidis, S., Dalekos, G. N., et al. (2021). An open label trial of anakinra to prevent respiratory failure in COVID-19. Immunology and Inflammation, Medicine, 10, e66125. https://doi.org/10.7554/eLife.66125
Bozzi, G., Mangioni, D., Minoia, F., Aliberti, S., Grasselli, G., Barbetta, L., et al. (2021). Anakinra Combined With Methylprednisolone in Patients With Severe COVID-19 Pneumonia and Hyperinflammation: An Observational Cohort Study. The Journal of Allergy and Clinical Immunology, 147(2), 561–564. https://doi.org/10.1016/j.jaci.2020.11.006
Franzetti, M., Forastieri, A., Borsa, N., Pandolfo, A., Molteni, C., Borghesi, L., et al. (2021). IL-1 Receptor Antagonist Anakinra in the Treatment of COVID-19 Acute Respiratory Distress Syndrome: A Retrospective. Observational Study. J. Immunol., 206(7), 1569–1575. https://doi.org/10.4049/jimmunol.2001126
Wang, Y., Zhu, K., Dai, R., et al. (2022). Specific Interleukin-1 Inhibitors, Specific Interleukin-6 Inhibitors, and GM-CSF Blockades for COVID-19 (at the Edge of Sepsis): A Systematic Review. Frontiers in Pharmacology, 12, 804250. https://doi.org/10.3389/fphar.2021.804250
Feldstein, L. R., Rose, E. B., Horwitz, S. M., Collins, J. P., Newhams, M. M., Son, M. B. F., et al. (2020). Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. New England Journal of Medicine, 383, 334–346. https://doi.org/10.1056/NEJMoa2021680
Pasin, L., Cavalli, G., Navalesi, P., Sella, N., Landoni d G, Yavorovskiy AG, et al. (2021). Anakinra for Patients With COVID-19: A Meta-Analysis of Non-Randomized Cohort Studies. Eur J Internal Med, 86, 34–40. https://doi.org/10.1016/j.ejim.2021.01.016
Monteagudo, L. A., Boothby, A., & Gertner, E. (2020). Continuous Intravenous Anakinra Infusion to Calm the Cytokine Storm in Macrophage Activation Syndrome. ACR Open Rheumatol, 2(5), 276–282. https://doi.org/10.1002/acr2.11135
Giavridis, T., van der Stegen, S. J., Eyquem, J., Hamieh, M., Piersigilli, A., & Sadelain, M. (2018). CAR T cell–induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nature Medicine, 24, 731–738. https://doi.org/10.1038/s41591-018-0041-7
Obstfeld, A. E., Frey, N. V., Mansfield, K., Lacey, S. F., June, C. H., & Porter, D. L. (2017). Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: Clinicopathological insights. Blood, 130, 2569–2572. https://doi.org/10.1182/blood-2017-08-802413
England, J. T., Abdulla, A., Biggs, C. M., et al. (2021). Weathering the COVID-19 storm: Lessons from hematologic cytokine syndromes. Blood Reviews, 45, 100707. https://doi.org/10.1016/j.blre.2020.100707
Fleischmann, R. M., Tesser, J., & Schiff, M. H. (2006). Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Annals of the Rheumatic Diseases, 65, 1006–1012.
Cavalli, G., & Dinarello, C. A. (2018). Anakinra therapy for non-cancer inflammatory diseases. Frontiers in Pharmacology, 9, 1157. https://doi.org/10.3389/fphar.2018.01157
Muir, R., Osbourn, M., Dubois, A. V., Doran, E., & Small, D. M. (2016). Innate lymphoid cells are the predominant source of IL-17A during the early pathogenesis of acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine, 193(4), 407–416. https://doi.org/10.1164/rccm.201410-1782OC PMID: 26488187.
Xu, Z., Shi, L., Wang, Y., Zhang, J., & Huang, L. (2020). Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine, 8(4), 420–422. https://doi.org/10.1016/S2213-2600(20)30076-X
Messina, F., & Piaserico, S. (2020). SARS-CoV-2 infection in a psoriatic patient treated with IL-23 inhibitor. Journal of the European Academy of Dermatology and Venereology, 34(6), e254–e255. https://doi.org/10.1111/jdv.16468
Raucci, F., Mansour, A. A., Casillo, G. M., Saviano, A., & Caso, F. (2020). Interleukin-17A (IL-17A), a key molecule of innate and adaptive immunity, and its potential involvement in COVID-19-related thrombotic and vascular mechanisms. Autoimmunity Reviews, 19(7), 102572. https://doi.org/10.1016/j.autrev.2020.102572
D’Antiga, L. (2020). Coronaviruses and immunosuppressed patients: The facts during the third epidemic. Liver Transplantation. https://doi.org/10.1002/It.25756
Gisondi, P., Facheris, P., Dapavo, P., Piaserico, S., & Conti, A. (2020). The impact of the COVID-19 pandemic on patients with chronic plaque psoriasis being treated with biological therapy: The Northern Italy experience. British Journal of Dermatology, 183(2), 373–374. https://doi.org/10.1111/bjd.19158
Yannam, G. R., Gutti, T., & Poluektova, L. Y. (2012). IL-23 in infections, inflammation, autoimmunity and cancer: Possible role in HIV-1 and AIDS. Journal of Neuroimmune Pharmacology, 7, 95–112. https://doi.org/10.1007/s11481-011-9315-2
Wu, D., & Yang, X. O. (2020). TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. Journal of Microbiology, Immunology, and Infection, 53(3), 368–370. https://doi.org/10.1016/j.jmii.2020.03.005
Xu, Z., Shi, L., Wang, Y., et al. (2020). Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine, 8(4), 420–422. https://doi.org/10.1016/S2213-2600(20)30076-X
Wang, C. J., & Truong, A. K. (2020). COVID-19 infection on IL-23 inhibition. Dermatologic Therapy, 33(6), e13893. https://doi.org/10.1111/dth.13893
Promising preclinical cancer therapy harnesses a newly discovered cell death pathway (2021) www.stjude.org. Accessed April 2, 2022
The PANoptosome: a new frontier in innate immune responses (2021) www.stjude.org. Accessed April 2, 2022
Karki, R., Sharma, B. R., Tuladhar, S., Williams, E. P., Zalduondo, L., Samir, P., et al. (2020). COVID-19 cytokines and the hyperactive immune response: Synergism of TNF-α and IFN-γ in triggering inflammation, tissue damage, and death. Cell, 184(1), 149–168. https://doi.org/10.1016/j.cell.2020.11.025
Gianfrancesco, M., Hyrich, K. L., Al-Adely, S., Carmona, L., Danila, M. I., Gossec, L., Izadi, Z., et al. (2020). Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: Data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Annals of the Rheumatic Diseases, 79, 859–866.
Mahil, S. K., Dand, N., Mason, K. J., Yiu, Z. Z. N., Tsakok, T., Meynell, F., Coker, B., McAteer, H., Moorhead, L., & Mackenzie, T. (2020). Factors associated with adverse COVID-19 outcomes in patients with psoriasis-insights from a global registry-based study. The Journal of Allergy and Clinical Immunology, 147(1), 60–71. https://doi.org/10.1016/j.jaci.2020.10.007
Brenner, E. J., Ungaro, R. C., Gearry, R. B., Kaplan, G. G., Kissous-Hunt, M., Lewis, J. D., Ng, S. C., Rahier, J.-F., Reinisch, W., & Ruemmele, F. M. (2020). Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: Results from an international registry. Gastroenterology, 159, 481–491. https://doi.org/10.1053/j.gastro.2020.05.032
Waggershauser, C. H., Tillack-Schreiber, C., Berchtold-Benchieb, C., Szokodi, D., Howaldt, S., & Ochsenkühn, T. (2020). Letter: Immunotherapy in IBD patients in a SARS-CoV-2 endemic area. Alimentary Pharmacology & Therapeutics, 52, 898–899. https://doi.org/10.1111/apt.15897
Rodríguez-Lago, I., Ramírez de la Piscina, P., Elorza, A., Merino, O., Ortiz de Zárate, J., & Cabriada, J. L. (2020). Characteristics and prognosis of patients with inflammatory bowel disease during the SARS-CoV-2 pandemic in the Basque country (Spain). Gastroenterology, 159, 781–783. https://doi.org/10.1053/j.gastro.2020.04.043
Stallmach, A., Kortgen, A., Gonnert, F., Coldewey, S.M., Reuken, P., Bauer, M. (2020). Infliximab against severe COVID-19-induced cytokine storm syndrome with organ failurea cautionary case series. Crit Care 24: 444. https://doi.org/10.1186/s13054-020-03158-0
Esmaeil, F. (2021). Cytokine storm response to COVID-19 vaccinations. Journal of Cytokine Biology, 5(2), 34–35.
Furer, V., Rondaan, C., Heijstek, M. W., Agmon-Levin, N., van Assen, S., Bijl, M., et al. (2019). 2019 Update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis (2019), 79(1), 39–52. https://doi.org/10.1136/annrheumdis-2019-215882
Agenzia Italiana Del Farmaco (2020). AIFA e Istituto Nazionale per lo studio e la cura dei tumori di Napoli avviano uno studio per l’utilizzo di Tocilizumab nella malattia COVID 19. Agenzia Italiana Del Farmaco.https://www.aifa.gov.it/web/guest/-/aifa-e-istituto-nazionale-per-lo-studio-e-la-cura-dei-tumeri-di-napoli-avviano-uno-studio-per-l-utilizzo-di-tocilizumab-nella-malattia-covid-19. Accessed 18 March 2022.
la Repubblica (2020). Coronavirus, tocilizumab: buoni risultati su due pazienti gravi. Urgente un protocollo per estendere uso del farmaco. la Repubblica. https://www.repubblica.it/oncologia/news/2020/03/11/news/coronavirus_buoni_risultati_su_due_pazienti_gravi_con_tocilizumab_ora_serve_un_protocollo_per_estendere_l_uso_del_farmaco-250937051/. Accessed 18 March 2020.
Tharmarajah, E., Buazon, A., Patel, V., Hannah, J. R., Adas, M., et al. (2021). IL-6 inhibition in the treatment of COVID-19: A meta-analysis and meta-regression. Journal of Infection, 82(5), 178–185. https://doi.org/10.1016/j.jinf.2021.03.008
Della-Torre, E., Campochiaro, C., Cavalli, G., & at al. (2020). Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: An open-label cohort study. Annals of the Rheumatic Diseases, 79, 1277–1285. https://doi.org/10.1136/annrheumdis-2020-218122
Gritti G, Raimondi F, Ripamonti D. et al. (2020) Il-6 signalling pathway inactivation with siltuximab in patients with COVID-19 respiratory failure: an observational cohort study. Cold Spring Harbor Laboratory Press. https://www.medrxiv.org/content/, https://doi.org/10.1101/2020.04.01.20048561v4. Accessed 20 March 2022.
Caricchio, R., Abbate, A., Gordeev, I., Meng, J., Hsue, P. Y., Neogi, T., et al. (2021). Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: A randomized clinical trial. JAMA, 326(3), 230–239. https://doi.org/10.1001/jama.2021.9508
Salesi, M., Shojaie, B., Farajzadegan, Z., et al. (2021). TNF-α blockers showed prophylactic effects in preventing COVID-19 in patients with rheumatoid arthritis and seronegative spondyloarthropathies: A case–control study. Rheumatol Ther., 8, 1355–1370. https://doi.org/10.1007/s40744-021-00342-8
Sharma, A. (2021). Randomized trial drug controlled compendious transcriptome analysis supporting broad and phase specific therapeutic potential of multiple candidates in COVID-19. Cytokine, 148, 155719. https://doi.org/10.1016/j.cyto.2021.155719
Funding
BNL was supported by the Kazan Federal University Strategic Academic Leadership Program (PRIORITY-2030).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
No
Research Involving Humans and Animals Statement
No
Informed Consent
No
Funding Statement
No
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Molecular, Precision and Regenerative Medicine - 2021
Rights and permissions
About this article
Cite this article
Kirillova, A., Lado, A. & Blatt, N. Application of Monoclonal Antibody Drugs in Treatment of COVID-19: a Review. BioNanoSci. 12, 1436–1454 (2022). https://doi.org/10.1007/s12668-022-00997-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-022-00997-9