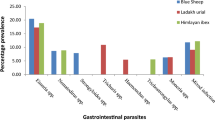
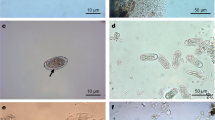
Abstract
Currently, a great proportion of the emerging infectious human diseases are zoonotic, with most of the pathogens originated from wildlife. In this sense, synanthropic animals such as marsupials play important role in the dissemination of pathogens due to their proximity to human dwellings. These hosts are affected by many gastrointestinal parasites, including species with zoonotic potential. The aim of this study was to assess the diversity of gastrointestinal parasites infecting the black-eared opossum D. aurita captured in urban areas of Southeastern, Brazil. In addition, the potential risk for the human population based on the One Health perspective has been discussed. Forty-nine marsupial specimens were captured with Tomahawk live traps and fecal samples were collected. The samples were evaluated by parasitological procedures. Eggs and oocysts were analyzed at different magnifications (400 × and 1000 ×), and their identification, together with adult nematodes, was established on morphological and morphometric data. Forty-three hosts (87.76%) scored positive for at least one gastrointestinal parasite, being 83.67% (41/49) for helminths, and 65.30% (32/49) for protozoa. For Cryptosporidium sp., only 13 samples were evaluated due to insufficient amount of feces obtained of some animals. A prevalence of 23.08% (3/13) was reported for this parasite. PCR analysis revealed Ancylostomatidae eggs to belong to the genus Ancylostoma. Our results demonstrated that multiparasitism is frequently found in these animals and a high percentage of potentially zoonotic parasites are observed, implying that D. aurita may be involved in zoonotic cycles in urban environments.




Similar content being viewed by others
References
Adnet FAO, Anjos DHS, Menezes-Oliveira A, Lanfredi RM (2009) Further description of Cruzia tentaculata (Rudolphi, 1819) Travassos, 1917 (Nematoda: Cruzidae) by light and scanning electron microscopy. Parasitol Res 104:1207–1211
Alden KJ (1995) Helminths of the opossum, Didelphis virginiana, in Southern Illinois, with a compilation of all helminths reported from this host in north America. J Helminthol Soc Wash 62(2):197–208
Anderson RC (2000) Nematode parasites of vertebrates. Their development and transmission. CABI Publishing, Wallingford
Aragón-Pech RA, Ruiz-Piña HÁ, Rodríguez-Vivas RI, Cuxim-Koyoc AD, Reyes-Novelo E (2018) Prevalence, abundance and intensity of eggs and oocysts of gastrointestinal parasites in the opossum Didelphis virginiana Kerr, 1792 in Yucatan, Mexico. Helminth 55(2):119–126
Batchelor DJ, Tzannes S, Graham PA, Wastling JM, Pinchbeck GL, German AJ (2008) Detection of endoparasites with zoonotic potential in dogs with gastrointestinal disease in the UK. Transbound Emerg Dis 55:99–104
Bermúdez SE, Gottdenker N, Krishnvajhala A, Fox A, Wilder HK, González K, Smith D, López M, Perea M, Rigg C, Montilla S, Calzada JE, Saldaña A, Caballero CM, Lopez JE (2017) Synanthropic mammals as potential hosts of tick-borne pathogens in panama. PLoS ONE 12(1):e0169047
Bouzid M, Hunter PR, Chalmers RM, Tyler KM (2013) Cryptosporidium Pathogenicity and Virulence. Clin Microbiol Rev 26(1):115–134
Bowman DD (2010) Georgis parasitologia veterinaria. Elsevier, Rio de Janeiro
Butcher AR, Grove DI (2001) Description of the life-cycle stages of Brachylaima cribbi n. sp. (Digenea: Brachylaimidae) derived from eggs recovered from human faeces in Australia. Syst Parasitol 49:211–221
Cáceres NC, Monteiro-Filho ELA (2001) Food Habits, Home Range and Activity of Didelphis aurita (Mammalia, Marsupialia) in a Forest Fragment of Southern Brazil. Stud Neotrop Fauna E 36(2):85–92
Cantor M, Ferreira LA, Silva WR, Setz EZF (2010) Potential seed dispersal by Didelphis albiventris (Marsupialia, Didelphidae) in highly disturbed environment. Biota Neotrop 10(2):45–51
Ceotto P, Finotti R, Santori R, Cerqueira R (2009) Diet variation of the marsupails Didelphis aurita and Philander frenatus (Didelphimorphia, Didelphidae) in a rural area of Rio de Janeiro State, Mexico. Mastozool Neotrop 16(1):49–58
Costa-Neto SF, Cardoso TS, Boullosa RG, Maldonado A Jr, Gentile R (2018) Metacommunity structure of the helminths of the black-eared opossum Didelphis aurita in peri-urban, sylvatic and rural environments in south-eastern Brazil. J Helminthol 17:1–12
Cutler SJ, Fooks AR, Van der Poel WHM (2010) Public health threat of new, reemerging, and neglected zoonoses in the industrialized world. Emerg Infect Dis 16(1):1–7
Duszynski DW (2016) The biology and identification of the coccidia (Apicomplexa) of marsupials of the world. Academic Press, Elsevier, San Diego and Waltham
Duszynski D, Wilber PG (1997) A guideline for the preparation of species descriptions in the eimeriidae. J Parasitol 83(2):333–336
Elliot A, Morgan UM, Aworew Thompson RC (1999) Improved staining method for detecting Cryptosporidium oocysts in stools using malachite green. J Gen Appl Microbiol 45:139–142
Ernst JV, Cooper C, Chobotar B (1969) Isospora boughtoni Volk, 1938 and Isospora sp. (Protozoa: Eimeridae) from an opossum Didelphis marsupialis. Bull Wildlife Dis 5:406–409
Feldmeier H, Schuster A (2012) Mini review: hookworm-related cutaneous larva migrans. Eur J Clin Microbiol Infect Dis 31:915–918
Furtado LF, Bello AC, Dos Santos HA, Carvalho MR, Rabelo ÉM (2014) First identification of the F200Y SNP in the β-tubulin gene linked to benzimidazole resistance in Ancylostoma caninum. Vet Parasitol 206(3–4):313–316
Gardner AL (2008) Mammals of South America, Volume I. Marsupials, xenarthrans, shrews, and bats. The University of Chicago Press, Chicago and London
Haverkost TR, Gardner SL (2008) A review of species in the genus Rhopalias (Rudolphi. J Parasitol 94(3):716–726
Joseph T (1974) Eimeria indianensis sp. n. and an Isospora sp. from the Opossum Didelphis virginiana (Kerr). J Protozool 21(1):12–15
Kawazoe U, Dias LCS, Piza JT (1978) Infecção natural de pequenos mamíferos por Schistosoma mansoni, na represa de Americana (São Paulo, Brasil). Rev Saúde Publ 12:200–208
Lainson R, Shaw JJ (1989) Two new species of Eimeria and three new species of Isospora Apicomplexa, Eimeriidae) from Brazilian mammals and birds. Bull Mus Hist Nat Paris 11(2):349–365
Lake SL, Matthews JB, Kaplan RM, Hodgkinson JE (2009) Determination of genomic DNA sequences for beta-tubulin isotype 1 from multiple species of cyathostomin and detection of resistance alleles in third-stage larvae from horses with naturally acquired infections. Parasit Vectors 2(2):S6
Lindsay DS, Dubey JP, Blagburn BL (1997) Biology of Isospora spp. from humans, nonhuman primates, and domestic animals. Clin Microbiol Rev 10(1):19–34
López-Caballero J, Mata-López R, De León GP (2019) Molecular data reveal a new species of Rhopalias Stiles & Hassall, 1898 (Digenea, Echinostomatidae) in the Common opossum, Didelphis marsupialis L. (Mammalia, Didelphidae) in the Yucatán Peninsula, Mexico. Zookey 854:145–163
Lucheis SB, Hernandes GS, Lenharo DK, Santiago MEB, Baldini-Peruca LC (2009) Are opossums capable of transmitting leptospirosis in urban areas? J Venom Anim Toxins Incl Trop Dis 15(3):370–373
Mateus TL, Castro A, Ribeiro JN, Vieira-Pinto M (2014) Multiple zoonotic parasites identified in dog feces collected in ponte de Lima, Portugal—a potential threat to human health. Int J Environ Res Public Health 11:9050–9067
McFarlane RO, Sleigh A, McMichael T (2012) Synanthropy of wild mammals as a determinant of emerging infectious diseases in the Asian–Australasian region. Eco Healt 9:24–35
Morgan JAT, Dejong RJ, Snyder SD, Mkoji GM, Loker ES (2001) Schistosoma mansoni and Biomphalaria: past history and future trends. Parasitol 123:S211–S228
Morrant DS, Petit S, Schumann R (2010) Floral nectar sugar composition and flowering phenology of the food plants used by the western pygmy possum, Cercartetus concinnus, at Innes National Park, South Australia. Ecol Res 25:579–589
Noronha D, Vicente JJ, Pinto RM (2002) A survey of new host records for nematodes from mammals deposited in the Helminthological Collection of the Oswaldo Cruz Institute (CHIOC). Rev Bras Zool 19(3):945–949
Oryan A, Sadjjadi SM, Mehrabani D, Kargar M (2008) Spirocercosis and its complications in stray dogs in Shiraz, southern Iran. Vet Med 53(11):617–624
Pereira AAS, Ferreira EC, Lima ACVMDR, Tonelli GB, Rêgo FD, Paglia AP, Andrade-Filho JD, Paz GF, Gontijo CMF (2017) Detection of Leishmania spp. in sylvatic mammals and isolation of Leishmania (Viannia) braziliensis from Rattus rattus in an endemic area for leishmaniasis in Minas Gerais State, Brazil. PLoS ONE 12(11):e0187704
Pinto CM, Ocaña-Mayorga S, Lascano MS, Grijalva MJ (2006) Infection by trypanosomes in marsupials and rodents associated with human dwellings in Ecuador. J Parasitol 92(6):1251–1255
Pinto HA, Mari VLT, Melo AL (2014) Toxocara cati (Nematoda: Ascarididae) in Didelphis albiventris (Marsupialia: Didelphidae) from Brazil: a case of pseudoparasitism. Braz J Vet Parasitol 23(4):522–525
Richardson DJ, Campo JD (2005) Gastrointestinal helminths of the virginia opossum (Didelphis virginiana) in South-Central Connecticut, USA. Comp Parasitol 72(2):183–185
Sahimin N, Lim YAL, Douadi B, Mohd Khalid MKN, Wilson JJ, Behnke JM, Mohd Zain SN (2017) Hookworm infections among migrant workers in Malaysia: Molecular identification of Necator americanus and Ancylostoma duodenale. Acta Trop 173:109–115
Teixeira M, Rauta PD, Albuquerque GR, Lopes CWG (2007) Eimeria auritanensis n. sp. and E. gambai Carini, 1938 (Apicomplexa: Eimeriidae) from the opossum Didelphis aurita Wied-newied, 1826 (Marsupialia: Didelphidae) from southeastern Brazil. Rev Bras Parasitol Vet 16(2):83–86
Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M (2010) Species interactions in a parasite community drive infection risk in a wildlife population. Scienc 330:243–246
Teodoro AKM, Cutolo AA, Motoie G, Meira-Strejevitch CS, Pereira-Chioccola VL, Mendes TMF, Allegretti SM (2019) Gastrointestinal, skin and blood parasites in Didelphis spp. from urban and sylvatic areas in São Paulo state, Brazil. Vet Parasitol Reg Stud Rep 16:100286
Thamsborg SM, Ketzis J, Horii Y, Matthews JB (2016) Strongyloides spp. infections of veterinary importance. Parasitol 144(3):274–284
Thompson RCA (2013) Parasite zoonoses and wildlife: one health, spillover and human activity. Int J Parasitol 43:1079–1088
Uehlinger FD, Greenwood SJ, McClure JT, Conboy G, O’Handley R, Barkema HW (2013) Zoonotic potential of Giardia duodenalis and Cryptosporidium spp. and prevalence of intestinal parasites in young dogs from different populations on Prince Edward Island, Canada. Vet Parasitol 196:509–514
Vaumourin E, Vourc’h G, Gasqui P, Vayssier-Taussat M (2015) The importance of multiparasitism: examining the consequences of coinfections for human and animal health. Parasite Vector 8:545
Viney ME, Lok JB (2015) The biology of Strongyloides spp. The C. elegans Research Community (ed) WormBook,. doi/https://doi.org/10.1895/wormbook.1.141.2, http://www.wormbook.org
Willis HH (1921) A simple levitation method for the detection of hookworm ova. Med J Aust 8:375–378
Youn H (2009) Review of zoonotic parasites in medical and veterinary fields in the Republic of Korea. Korean J Parasitol 47:S133–S141
Zanette RA, Silva AS, Lunardi F, Santurio JM, Monteiro SG (2008) Occurrence of gastrointestinal protozoa in Didelphis albiventris (opossum) in the central region of Rio Grande do Sul state. Parasitol Int 57(2):217–218
Zuccherato LW, Furtado LF, Medeiros CS, Pinheiro CS, Rabelo ÉM (2018) PCR-RFLP screening of polymorphisms associated with benzimidazole resistance in Necator americanus and Ascaris lumbricoides from different geographical regions in Brazil. PLoS Negl Trop Dis 12(9):e0006766
Acknowledgements
The authors acknowledge the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), and the Graduate Program in Veterinary Medicine of the Universidade Federal de Viçosa (UFV) for the support provided. Finally, authors would like to thank Professor Gisele M. L. del Giúdice for providing traps to capture the animals.
Author information
Authors and Affiliations
Contributions
MABS, CSF and BCFN collected samples and performed laboratory procedures. LFVF and EMLR performed the molecular procedures. AKC, RANR and RSY supervised the study. MABS, RSY and JAG analyzed the data. MABS wrote the first draft of the manuscript. AKC, RANR, RSY, LFVF, EMLR and JVA revised and performed corrections on the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The Ethics Committee for Animal Experimentation (ECAE) of the Universidade Federal de Viçosa (License Number: 80/2018) and the Biodiversity Information and Authorization System (SISBIO) (License Number: 64930-1) of the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) approved all procedures herein performed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bezerra-Santos, M.A., Fontes, C.S., Nogueira, B.C.F. et al. Gastrointestinal parasites in the opossum Didelphis aurita: Are they a potential threat to human health?. J Parasit Dis 44, 355–363 (2020). https://doi.org/10.1007/s12639-020-01205-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-020-01205-9