Abstract
Purpose
It has been suggested that a larger heparin dose during cardiopulmonary bypass (CPB) is associated with reduced perioperative coagulopathy and thromboembolic complications. We investigated the effect of different heparin doses during routine elective cardiac surgery. Our primary outcomes include blood loss and transfusion and secondary outcomes investigate the effects on coagulation biomarkers.
Methods
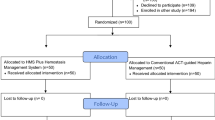
In this prospective pilot trial, we allocated 60 patients undergoing cardiac surgery on CPB in a single tertiary cardiac centre into three groups to receive an initial dose of 300, 400, or 500 units (U) per kilogram of intravenous heparin prior to the commencement of CPB. Blood was sampled after induction of anesthesia, at 30 and 60 min of CPB, and three minutes after heparin reversal with protamine. Samples were analyzed for fibrinopeptide A (FPA), fibrinopeptide B (FPB), D-dimer, and thrombin-antithrombin (TAT) complexes. Postoperative blood loss and transfusion was measured for the first 24-hr period after surgery.
Results
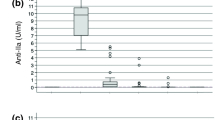
The total mean (95% CI) administered heparin dose in the 300 U·kg−1, 400 U·kg−1, and 500 U·kg−1 groups were 39,975 (36,528 to 43,421) U, 43,195 (36,940 to 49,449) U and 47,900 (44,807 to 50,992) U, respectively. There were no statistically significant differences in FPA, FPB or D-dimer levels at the measured time intervals. There was a trend towards lower TAT levels while on CPB with greater heparin dosing, which was statistically significant after the administration of protamine. The clinical significance appears to be negligible, as there is no difference in overall blood loss and amount of packed red blood cell transfusion or other blood product transfusion.
Conclusion
This pilot study indicates that, while larger heparin dosing for routine cardiac surgery results in subtle biochemical changes in coagulation, there is no demonstrable benefit in postoperative blood loss or transfusion requirements.
Résumé
Objectif
Il a été suggéré qu’une dose plus élevée d’héparine pendant la circulation extracorporelle (CEC) serait associée à une réduction de la coagulopathie périopératoire et des complications thromboemboliques. Nous avons étudié l’effet de différentes doses d’héparine au cours d’une chirurgie cardiaque non urgente de routine. Nos critères d’évaluation principaux comprenaient la perte de sang et la transfusion, et les critères d’évaluation secondaires exploraient les effets sur les biomarqueurs de la coagulation.
Méthode
Dans cette étude pilote prospective, nous avons réparti 60 patient·es bénéficiant d’une chirurgie cardiaque sous CEC dans un seul centre cardiaque tertiaire en trois groupes à recevoir une dose initiale de 300, 400 ou 500 unités (U) par kilogramme d’héparine intraveineuse avant le début de la CEC. Le sang a été prélevé après l’induction de l’anesthésie, à 30 et 60 minutes de CEC, et trois minutes après la neutralisation de l’héparine avec la protamine. Les échantillons ont été analysés pour les complexes fibrinopeptide A (FPA), fibrinopeptide B (FPB), D-dimère et thrombine-antithrombine (TAT). La perte de sang postopératoire et la transfusion ont été mesurées pendant la première période de 24 heures après la chirurgie.
Résultats
La dose moyenne totale (IC 95 %) d’héparine administrée dans les 300 U·kg−1, 400 U·kg−1, et 500 U·kg−1 était de 39 975 (36 528 à 43 421) U, 43 195 (36 940 à 49 449) U et 47 900 (44 807 à 50 992) U, respectivement. Il n’y avait aucune différence statistiquement significative dans les taux de FPA, FPB ou D-dimères aux intervalles de temps mesurés. Une tendance à des niveaux de TAT plus bas pendant la CEC a été observée avec une dose d’héparine plus élevée, ce qui était statistiquement significatif après l’administration de protamine. La signification clinique semble négligeable, car il n’y a pas de différence dans la perte de sang globale et la quantité de transfusion de concentrés globulaires ou d’autres produits sanguins.
Conclusion
Cette étude pilote indique que, bien qu’une dose plus importante d’héparine pour la chirurgie cardiaque de routine entraîne des changements biochimiques subtils dans la coagulation, il n’y a aucun avantage démontrable en matière de saignement postopératoire ou de besoins transfusionnels.

Similar content being viewed by others
References
Shore-Lesserson L, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and The American Society of ExtraCorporeal Technology: clinical practice guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg 2018; 105: 650–62. https://doi.org/10.1016/j.athoracsur.2017.09.061
Lax M, Pesonen E, Hiippala S, Schramko A, Lassila R, Raivio P. Heparin dose and point-of-care measurements of hemostasis in cardiac surgery-results of a randomized controlled trial. J Cardiothorac Vasc Anesth 2020; 34: 2362–8. https://doi.org/10.1053/j.jvca.2019.12.050
Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg 2002; 21: 232–44. https://doi.org/10.1016/s1010-7940(01)01099-5
Besser MW, Klein AA. The coagulopathy of cardiopulmonary bypass. Crit Rev Clin Lab Sci 2010; 47: 197–212. https://doi.org/10.3109/10408363.2010.549291
Falter F, MacDonald S, Matthews C, Kemna E, Cañameres J, Besser M. Evaluation of point-of-care ACT coagulometers and anti-Xa activity during cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2020; 34: 2921–7. https://doi.org/10.1053/j.jvca.2020.06.027
Miles LF, Coulson TG, Galhardo C, Falter F. Pump priming practices and anticoagulation in cardiac surgery: results from the global cardiopulmonary bypass survey. Anesth Analg 2017; 125: 1871–7. https://doi.org/10.1213/ane.0000000000002052
Despotis GJ, Joist JH, Hogue CW Jr, et al. More effective suppression of hemostatic system activation in patients undergoing cardiac surgery by heparin dosing based on heparin blood concentrations rather than ACT. Thromb Haemost 1996; 76: 902–8.
Okita Y, Takamoto S, Ando M, et al. Coagulation and fibrinolysis system in aortic surgery under deep hypothermic circulatory arrest with aprotinin: the importance of adequate heparinization. Circulation 1997; 96: 376–81.
Hirsh J, Anand SS, Halperin JL, Fuster V, American Heart Association. AHA scientific statement: guide to anticoagulant therapy: heparin: a statement for healthcare professionals from the American Heart Association. Arterioscler Thromb Vasc Biol 2001; 21: E9. https://doi.org/10.1161/hq0701.093520
Miles LF, Burt C, Arrowsmith J, et al. Optimal protamine dosing after cardiopulmonary bypass: the PRODOSE adaptive randomised controlled trial. PLoS Med 2021; 18: e1003658. https://doi.org/10.1371/journal.pmed.1003658
Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res 2016; 25: 1057–73. https://doi.org/10.1177/0962280215588241
Boisclair MD, Lane DA, Philippou H, Sheikh S, Hunt B. Thrombin production, inactivation and expression during open heart surgery measured by assays for activation fragments including a new ELISA for prothrombin fragment F1 + 2. Thromb Haemost 1993; 70: 253–8.
Brister SJ, Ofosu FA, Buchanan MR. Thrombin generation during cardiac surgery: is heparin the ideal anticoagulant? Thromb Haemost 1993; 70: 259–62.
Apte G, Börke J, Rothe H, Liefeith K, Nguyen TH. Modulation of platelet-surface activation: current state and future perspectives. ACS Appl Bio Mater 2020; 3: 5574–89. https://doi.org/10.1021/acsabm.0c00822
Koster A, Yeter R, Buz S, et al. Assessment of hemostatic activation during cardiopulmonary bypass for coronary artery bypass grafting with bivalirudin: results of a pilot study. J Thorac Cardiovasc Surg 2005; 129: 1391–4. https://doi.org/10.1016/j.jtcvs.2004.09.016
Lander H, Zammert M, FitzGerald D. Anticoagulation management during cross-clamping and bypass. Best Pract Res Clin Anaesthesiol 2016; 30: 359–70. https://doi.org/10.1016/j.bpa.2016.07.002
Garvin S, Fitzgerald D, Muehlschlegel JD, et al. Heparin dose response is independent of preoperative antithrombin activity in patients undergoing coronary artery bypass graft surgery using low heparin concentrations. Anesth Analg 2010; 111: 856–61. https://doi.org/10.1213/ane.0b013e3181ce1ffa
Shuhaibar MN, Hargrove M, Millat MH, O'Donnell A, Aherne T. How much heparin do we really need to go on pump? A rethink of current practices. Eur J Cardiothorac Surg 2004; 26: 947–50. https://doi.org/10.1016/j.ejcts.2004.07.009
Bull BS, Korpman RA, Huse WM, Briggs BD. Heparin therapy during extracorporeal circulation. I. Problems inherent in existing heparin protocols. J Thorac Cardiovasc Surg 1975; 69: 674–84.
Palmer K, Ridgway T, Al-Rawi O, Poullis M. Heparin therapy during extracorporeal circulation: deriving an optimal activated clotting time during cardiopulmonary bypass for isolated coronary artery bypass grafting. J Extra Corpor Technol 2012; 44: 145–50.
Delavenne X, Ollier E, Chollet S, et al. Pharmacokinetic/pharmacodynamic model for unfractionated heparin dosing during cardiopulmonary bypass. Br J Anaesth 2017; 118: 705–12. https://doi.org/10.1093/bja/aex044
Sniecinski RM, Chandler WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg 2011; 113: 1319–33. https://doi.org/10.1213/ane.0b013e3182354b7e
Chandler WL, Velan T. Plasmin generation and D-dimer formation during cardiopulmonary bypass. Blood Coagul Fibrinolysis 2004; 15: 583–91. https://doi.org/10.1097/00001721-200410000-00009
Nossel HL, Ti M, Kaplan KL, Spanondis K, Soland T, Butler VP Jr. The generation of fibrinopeptide A in clinical blood samples: evidence for thrombin activity. J Clin Invest 1976; 58: 1136–44. https://doi.org/10.1172/jci108566
Davies GC, Sobel M, Salzman EW. Elevated plasma fibrinopeptide A and thromboxane B2 levels during cardiopulmonary bypass. Circulation 1980; 61: 808–14. https://doi.org/10.1161/01.cir.61.4.808
Muedra V, Bonanad S, Gómez M, Villalonga V, Sánchez F, Llopis JE. Relationships between antithrombin activity, anticoagulant efficacy of heparin therapy and perioperative variables in patients undergoing cardiac surgery requiring cardiopulmonary bypass. Perfusion 2011; 26: 487–95. https://doi.org/10.1177/0267659111412999
Chandler WL, Velan T. Estimating the rate of thrombin and fibrin generation in vivo during cardiopulmonary bypass. Blood 2003; 101: 4355–62. https://doi.org/10.1182/blood-2002-08-2400
Sato H, Yamamoto K, Kakinuma A, Nakata Y, Sawamura S. Accelerated activation of the coagulation pathway during cardiopulmonary bypass in aortic replacement surgery: a prospective observational study. J Cardiothorac Surg 2015; 10: 84. https://doi.org/10.1186/s13019-015-0295-9
Riedel T, Suttnar J, Brynda E, Houska M, Medved L, Dyr JE. Fibrinopeptides A and B release in the process of surface fibrin formation. Blood 2011; 117: 1700–6. https://doi.org/10.1182/blood-2010-08-300301
Rimpo K, Tanaka A, Ukai M, Ishikawa Y, Hirabayashi M, Shoyama T. Thrombin-antithrombin complex measurement using a point-of-care testing device for diagnosis of disseminated intravascular coagulation in dogs. PLoS One 2018; 13: e0205511. https://doi.org/10.1371/journal.pone.0205511
Ruhl H, Berens C, Winterhagen A, Müller J, Oldenburg J, Potzsch B. Label-free kinetic studies of hemostasis-related biomarkers including D-dimer using autologous serum transfusion. PLoS One 2015; 10: e0145012. https://doi.org/10.1371/journal.pone.0145012
Eisenberg PR, Lucore C, Kaufman L, Sobel BE, Jaffe AS, Rich S. Fibrinopeptide A levels indicative of pulmonary vascular thrombosis in patients with primary pulmonary hypertension. Circulation 1990; 82: 841–7. https://doi.org/10.1161/01.cir.82.3.841
Ainle FN, Preston RJ, Jenkins PV, et al. Protamine sulfate down-regulates thrombin generation by inhibiting factor V activation. Blood 2009; 114: 1658–65. https://doi.org/10.1182/blood-2009-05-222109
Meesters MI, Veerhoek D, de Jong JR, Boer C. A Pharmacokinetic model for protamine dosing after cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2016; 30: 1190–5. https://doi.org/10.1053/j.jvca.2016.04.021
Goedhart AL, Gerritse BM, Rettig TC, et al. A 0.6-protamine/heparin ratio in cardiac surgery is associated with decreased transfusion of blood products. Interact Cardiovasc Thorac Surg 2020; 31: 391–7. https://doi.org/10.1093/icvts/ivaa109
Mahmood S, Bilal H, Zaman M, Tang A. Is a fully heparin-bonded cardiopulmonary bypass circuit superior to a standard cardiopulmonary bypass circuit? Interact Cardiovasc Thorac Surg 2012; 14: 406–14. https://doi.org/10.1093/icvts/ivr124
Author contributions
Thar Nyan Lwin and Rahul Mudannayake contributed to data curation, investigation, and writing the original draft and edited versions. Stephen MacDonald contributed to laboratory and data analysis, writing the original draft, and reviewing and editing the manuscript. Joseph Arrowsmith and Christiana Burt contributed to data curation and investigation. Martin Besser contributed to supervision, formal analysis, and reviewed and edited the manuscript. Florian Falter contributed to conceptualization, data curation, investigation, formal analysis, supervision, and reviewed and edited the manuscript.
Acknowledgements
We thank Dr Martin Law form the MRC Cambridge Biostatistics Unit for reviewing the statistical calculations.
Disclosures
Martin Besser receives honoraria, educational support and grants from Sanofi, Novartis, Griffols, Novartis, GBT, Amgen. He is a member of advisory boards for Novartis, GBT, Forma, Amgen, Hemeo, Octapharma. Florian Falter receives honoraria from Abbott Point of Care and Werfen. He is a member of advisory boards for Abbott Diabetics, Abbott Point of Care and Werfen. No other authors have any disclosures to make.
Funding statement
No funding was received.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Deputy Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lwin, T.N., Mudannayake, R., MacDonald, S. et al. Assessing the impact of different heparin dosing regimens for cardiopulmonary bypass on anticoagulation: the HepDOSE pilot study. Can J Anesth/J Can Anesth 71, 234–243 (2024). https://doi.org/10.1007/s12630-023-02645-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02645-6