Abstract
Purpose
Cold-stored platelets (CSP) are an increasingly active topic of international research. They are maintained at 1–6 °C, in contrast to standard room-temperature platelets (RTP) kept at 20–24 °C. Recent evidence suggests that CSP have superior hemostatic properties compared with RTP. This narrative review explores the application of CSP in adult cardiac surgery, summarizes the preclinical and clinical evidence for their use, and highlights recent research.
Source
A targeted search of MEDLINE and other databases up to 24 February 2022 was conducted. Search terms combined concepts such as cardiac surgery, blood, platelet, and cold-stored. Searches of trial registries ClinicalTrials.gov and WHO International Clinical Trials Registry Platform were included. Articles were included if they described adult surgical patients as their population of interest and an association between CSP and clinical outcomes. References of included articles were hand searched.
Principal findings
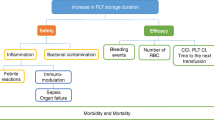
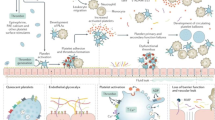
When platelets are stored at 1–6 °C, their metabolic rate is slowed, preserving hemostatic function for increased storage duration. Cold-stored platelets have superior adhesion characteristics under physiologic shear conditions, and similar or superior aggregation responses to physiologic agonists. Cold-stored platelets undergo structural, metabolic, and molecular changes which appear to “prime” them for hemostatic activity. While preliminary, clinical evidence supports the conduct of trials comparing CSP with RTP for patients with platelet-related bleeding, such as those undergoing cardiac surgery.
Conclusion
Cold-stored platelets may have several advantages over RTP, including increased hemostatic capacity, extended shelf-life, and reduced risk of bacterial contamination. Large clinical trials are needed to establish their potential role in the treatment of acutely bleeding patients.
Résumé
Objectif
Les plaquettes conservées au froid (PCF) sont un sujet de recherche internationale de plus en plus populaire. Ces plaquettes sont maintenues à une température de 1-6 °C, contrairement aux plaquettes standard conservées à température ambiante (PTA), maintenues à 20–24 °C. Des données probantes récentes suggèrent que les PCF ont des propriétés hémostatiques supérieures aux PTA. Ce compte rendu narratif explore l’application de PCF en chirurgie cardiaque chez l’adulte, résume les données probantes précliniques et cliniques de leur utilisation, et met en évidence les recherches récentes.
Sources
Une recherche ciblée dans MEDLINE et d’autres bases de données jusqu’au 24 février 2022 a été effectuée. Les termes de recherche combinaient des concepts en anglais tels que cardiac surgery, blood, platelet et cold-stored (soit chirurgie cardiaque, plaquette, et entreposage frigorifique). Des recherches dans les registres d’études ClinicalTrials.gov et le système d’enregistrement international des essais cliniques (ICTRP) de l’OMS ont été incluses. Les articles ont été inclus s’ils décrivaient des patient·es adultes de chirurgie en tant que population d’intérêt et une association entre les PCF et les issues cliniques. Les références des articles inclus ont fait l’objet d’une recherche manuelle.
Constatations principales
Lorsque les plaquettes sont conservées entre 1 et 6 °C, leur taux métabolique est ralenti, préservant la fonction hémostatique pour une durée d’entreposage accrue. Les plaquettes conservées au froid ont des caractéristiques d’adhésion supérieures dans des conditions de cisaillement physiologique et des réponses d’agrégation similaires ou supérieures aux agonistes physiologiques. Les plaquettes conservées au froid subissent des changements structurels, métaboliques et moléculaires qui semblent les « amorcer » pour une activité hémostatique. Bien que préliminaires, les données probantes cliniques appuient la réalisation d’études comparant les PCF aux PTA chez la patientèle présentant des saignements liés aux plaquettes, tels que les personnes bénéficiant d’une chirurgie cardiaque.
Conclusion
Les plaquettes conservées au froid peuvent présenter plusieurs avantages par rapport aux PTA, notamment une capacité hémostatique accrue, une durée de conservation prolongée et un risque réduit de contamination bactérienne. De grands essais cliniques sont nécessaires pour établir leur rôle potentiel dans le traitement de la patientèle en hémorragie aiguë.

Similar content being viewed by others
References
Holinstat M. Normal platelet function. Cancer Metastasis Rev 2017; 36: 195–8. https://doi.org/10.1007/s10555-017-9677-x
Bartoszko J, Karkouti K. Managing the coagulopathy associated with cardiopulmonary bypass. J Thromb Haemost 2021; 19: 617–32. https://doi.org/10.1111/jth.15195
Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion 2016; 56: 2173–83. https://doi.org/10.1111/trf.13676
Webert KE, Alam AQ, Chargé SB, Sheffield WP. Platelet utilization: a Canadian Blood Services research and development symposium. Transfus Med Rev 2014; 28: 84–97. https://doi.org/10.1016/j.tmrv.2014.01.002
Jones JM, Sapiano MRP, Mowla S, Bota D, Berger JJ, Basavaraju SV. Has the trend of declining blood transfusions in the United States ended? Findings of the 2019 National Blood Collection and Utilization Survey. Transfusion 2021; 61: S1–10. https://doi.org/10.1111/trf.16449
Ontario Regional Blood Coordinating Network. Provincial platelet audit report (audit period January 9 - April 7, 2017), 2017. Available from URL: https://transfusionontario.org/wp-content/uploads/2020/06/Plt-Audit-Report_final-1-2.pdf (accessed May 2023).
Estcourt LJ. Why has demand for platelet components increased? A review. Transfus Med 2014; 24: 260–8. https://doi.org/10.1111/tme.12155
Estcourt LJ, Birchall J, Allard S, et al. Guidelines for the use of platelet transfusions. Br J Haematol 2017; 176: 365–94. https://doi.org/10.1111/bjh.14423
Mowla SJ, Sapiano MRP, Jones JM, Berger JJ, Basavaraju SV. Supplemental findings of the 2019 National Blood Collection and Utilization Survey. Transfusion 2021; 61: S11–35. https://doi.org/10.1111/trf.16606
Rogers MA, Greene MT, Davis JA, et al. Longitudinal study of transfusion utilization in hospitalized veterans. J Clin Outcomes Manag 2017; 24: 404–11. http://www.ncbi.nlm.nih.gov/pmc/articles/pmc6072265/
Shehata N, Forster A, Lawrence N, et al. Changing trends in blood transfusion: an analysis of 244,013 hospitalizations. Transfusion 2014; 54: 2631–9. https://doi.org/10.1111/trf.12644
Ramirez-Arcos S, Evans S, McIntyre T, et al. Extension of platelet shelf life with an improved bacterial testing algorithm. Transfusion 2020; 60: 2918–28. https://doi.org/10.1111/trf.16112
Harris JC, Crookston KP. Blood product safety, 2019. Available from URL: https://europepmc.org/article/MED/30969648/NBK430685#free-full-text (accessed May 2023).
Warner MA, Kurian EB, Hammel SA, van Buskirk CM, Kor DJ, Stubbs JR. Transition from room temperature to cold-stored platelets for the preservation of blood inventories during the COVID-19 pandemic. Transfusion 2021; 61: 72–7. https://doi.org/10.1111/trf.16148
International Society of Blood Transfusion. The 36th International ISBT Congress, virtual meeting, 12-16 December 2020. Vox Sang 2020; 115: 5–396. https://doi.org/10.1111/vox.13031
Chin V, Cope S, Yeh CH, et al. Massive hemorrhage protocol survey: marked variability and absent in one-third of hospitals in Ontario, Canada. Injury 2019; 50: 46–53. https://doi.org/10.1016/j.injury.2018.11.026
Aubron C, Flint AWJ, Ozier Y, McQuilten Z. Platelet storage duration and its clinical and transfusion outcomes: a systematic review. Crit Care 2018; 22: 185. https://doi.org/10.1186/s13054-018-2114-x
Becker GA, Tuccelli M, Kunicki T, Chalos MK, Aster RH. Studies of platelet concentrates stored at 22 C nad 4 C. Transfusion 1973; 13: 61–8. https://doi.org/10.1111/j.1537-2995.1973.tb05442.x
Zhao H, Devine DV. The missing pieces to the cold-stored platelet puzzle. Int J Mol Sci 2022; 23: 1100. https://doi.org/10.3390/ijms23031100
Baharoglu MI, Cordonnier C, Al-Shahi Salman R, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet 2016; 387: 2605–13. https://doi.org/10.1016/s0140-6736(16)30392-0
Estcourt LJ. Platelet transfusion thresholds in premature neonates (PlaNeT-2 trial). Transfus Med 2019; 29: 20–2. https://doi.org/10.1111/tme.12587
Strandenes G, Sivertsen J, Bjerkvig CK, et al. A pilot trial of platelets stored cold versus at room temperature for complex cardiothoracic surgery. Anesthesiology 2020; 133: 1173–83. https://doi.org/10.1097/aln.0000000000003550
Braathen H, Sivertsen J, Lunde TH, et al. In vitro quality and platelet function of cold and delayed cold storage of apheresis platelet concentrates in platelet additive solution for 21 days. Transfusion 2019; 59: 2652–61. https://doi.org/10.1111/trf.15356
Krachey E, Viele K, Spinella PC, Steiner ME, Zantek ND, Lewis RJ. The design of an adaptive clinical trial to evaluate the efficacy of platelets stored at low temperature in surgical patients. J Trauma Acute Care Surg 2018; 84: S41–6. https://doi.org/10.1097/ta.0000000000001876
Slichter SJ, Fitzpatrick L, Osborne B, et al. Platelets stored in whole blood at 4°C: in vivo posttransfusion platelet recoveries and survivals and in vitro hemostatic function. Transfusion 2019; 59: 2084–92. https://doi.org/10.1111/trf.15302
Charlton A, Wallis J, Robertson J, Watson D, Iqbal A, Tinegate H. Where did platelets go in 2012? A survey of platelet transfusion practice in the North of England. Transfus Med 2014; 24: 213–8. https://doi.org/10.1111/tme.12126
Apelseth TO, Cap AP, Spinella PC, Hervig T, Strandenes G. Cold stored platelets in treatment of bleeding. ISBT Sci Series 2017; 12: 488–95. https://doi.org/10.1111/voxs.12380
Orlov D, McCluskey SA, Selby R, Yip P, Pendergrast J, Karkouti K. Platelet dysfunction as measured by a point-of-care monitor is an independent predictor of high blood loss in cardiac surgery. Anesth Analg 2014; 118: 257–63. https://doi.org/10.1213/ane.0000000000000054
Ranucci M, Pistuddi V, Di Dedda U, Menicanti L, De Vincentiis C, Baryshnikova E. Platelet function after cardiac surgery and its association with severe postoperative bleeding: the PLATFORM study. Platelets 2019; 30: 908–14. https://doi.org/10.1080/09537104.2018.1535706
Braathen H, Hagen KG, Kristoffersen EK, Strandenes G, Apelseth TO. Implementation of a dual platelet inventory in a tertiary hospital during the COVID-19 pandemic enabling cold-stored apheresis platelets for treatment of actively bleeding patients. Transfusion 2022; 62: S193–202. https://doi.org/10.1111/trf.16988
Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev 2019; 4: 5. https://doi.org/10.1186/s41073-019-0064-8
Association for the Advancement of Blood & Biotherapies. Highlights of transfusion medicine history, 2023. Available from URL: https://www.aabb.org/news-resources/resources/transfusion-medicine/highlights-of-transfusion-medicine-history (accessed May 2023).
Cap AP, Beckett A, Benov A, et al. Whole blood transfusion. Mil Med 2018; 183: 44–51. https://doi.org/10.1093/milmed/usy120
Kogler VJ, Stolla M. There and back again: the once and current developments in donor-derived platelet products for hemostatic therapy. Blood 2022; 139: 3688–98. https://doi.org/10.1182/blood.2021014889
Silva VA, Miller WV. Platelet transfusion survey in a regional blood program. Transfusion 1977; 17: 255–60. https://doi.org/10.1046/j.1537-2995.1977.17377196361.x
Levin RH, Freireich EJ, Chappell W. Effect of storage up to 48 hours on response to transfusions of platelet rich plasma. Transfusion 1964; 4: 251–6. https://doi.org/10.1111/j.1537-2995.1964.tb02867.x
Murphy S, Gardner FH. Effect of storage temperature on maintenance of platelet viability—deleterious effect of refrigerated storage. N Engl J Med 1969; 280: 1094–8. https://doi.org/10.1056/nejm196905152802004
Slichter SJ, Harker LA. Preparation and storage of platelet concentrates. II. Storage variables influencing platelet viability and function. Br J Haematol 1976; 34: 403–19. https://doi.org/10.1111/j.1365-2141.1976.tb03587.x
Slichter SJ, Harker LA. Preparation and storage of platelet concentrates. Transfusion 1976; 16: 8–12. https://doi.org/10.1046/j.1537-2995.1976.16176130842.x
Valeri CR. Hemostatic effectiveness of liquid-preserved and previously frozen human platelets. N Engl J Med 1974; 290: 353–8. https://doi.org/10.1056/nejm197402142900702
Handin RI, Valeri CR. Hemostatic effectiveness of platelets stored at 22 degrees C. N Engl J Med 1971; 285: 538–43. https://doi.org/10.1056/nejm197109022851003
Valeri CR. Circulation and hemostatic effectiveness of platelets stored at 4 C or 22 C: studies in aspirin-treated normal volunteers. Transfusion 1976; 16: 20–3. https://doi.org/10.1046/j.1537-2995.1976.16176130832.x
Shea SM, Thomas KA, Spinella PC. The effect of platelet storage temperature on haemostatic, immune, and endothelial function: potential for personalised medicine. Blood Transfus 2019; 17: 321–30. https://doi.org/10.2450/2019.0095-19
Bailey SL, Fang LY, Fitzpatrick L, Byrne D, Pellham E, Stolla M. In vitro and in vivo effects of short-term cold storage of platelets in PAS-C. Haematologica 2022; 107: 988–90. https://doi.org/10.3324/haematol.2021.279865
Mack JP, Miles J, Stolla M. Cold-stored platelets: review of studies in humans. Transfus Med Rev 2020; 34: 221–6. https://doi.org/10.1016/j.tmrv.2020.08.003
van der Wal DE, Du VX, Lo KS, Rasmussen JT, Verhoef S, Akkerman JW. Platelet apoptosis by cold-induced glycoprotein Ibα clustering. J Thromb Haemost 2010; 8: 2554–62. https://doi.org/10.1111/j.1538-7836.2010.04043.x
van der Wal DE, Gitz E, Du VX, et al. Arachidonic acid depletion extends survival of cold-stored platelets by interfering with the [glycoprotein Ibα--14-3-3ζ] association. Haematologica 2012; 97: 1514–22. https://doi.org/10.3324/haematol.2011.059956
Quach ME, Chen W, Li R. Mechanisms of platelet clearance and translation to improve platelet storage. Blood 2018; 131: 1512–21. https://doi.org/10.1182/blood-2017-08-743229
Rumjantseva V, Grewal PK, Wandall HH, et al. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med 2009; 15: 1273–80. https://doi.org/10.1038/nm.2030
Jansen AJ, Josefsson EC, Rumjantseva V, et al. Desialylation accelerates platelet clearance after refrigeration and initiates GPIbα metalloproteinase-mediated cleavage in mice. Blood 2012; 119: 1263–73. https://doi.org/10.1182/blood-2011-05-355628
Hoffmeister KM, Felbinger TW, Falet H, et al. The clearance mechanism of chilled blood platelets. Cell 2003; 112: 87–97. https://doi.org/10.1016/s0092-8674(02)01253-9
Wandall HH, Hoffmeister KM, Sørensen AL, et al. Galactosylation does not prevent the rapid clearance of long-term, 4 degrees C-stored platelets. Blood 2008; 111: 3249–56. https://doi.org/10.1182/blood-2007-06-097295
Lozano M. Platelets come back in from the cold. Blood 2008; 111: 2951. https://doi.org/10.1182/blood-2008-01-131920
Hoffmeister KM, Falet H, Toker A, Barkalow KL, Stossel TP, Hartwig JH. Mechanisms of cold-induced platelet actin assembly. J Biol Chem 2001; 276) :24751–9.
Oliver AE, Tablin F, Walker NJ, Crowe JH. The internal calcium concentration of human platelets increases during chilling. Biochim Biophys Acta 1999; 1416: 349–60. https://doi.org/10.1016/s0005-2736(98)00239-9
Hartwig JH. Mechanisms of actin rearrangements mediating platelet activation. J Cell Biol 1992; 118: 1421–2. https://doi.org/10.1083/jcb.118.6.1421
Johnson L, Tan S, Wood B, Davis A, Marks DC. Refrigeration and cryopreservation of platelets differentially affect platelet metabolism and function: a comparison with conventional platelet storage conditions. Transfusion 2016; 56: 1807–18. https://doi.org/10.1111/trf.13630
Johnson L, Vekariya S, Wood B, Tan S, Roan C, Marks DC. Refrigeration of apheresis platelets in platelet additive solution (PAS-E) supports in vitro platelet quality to maximize the shelf-life. Transfusion 2021; 61: S58–67. https://doi.org/10.1111/trf.16489
Zhao HQ, Serrano K, Culibrk B, Chen Z, Devine DV. Cold-stored platelets are effective in an in vitro model of massive transfusion protocol assessed by rotational thromboelastometry. Transfusion 2022; 62: S53–62. https://doi.org/10.1111/trf.16974
Bynum JA, Meledeo MA, Getz TM, et al. Bioenergetic profiling of platelet mitochondria during storage: 4°C storage extends platelet mitochondrial function and viability. Transfusion 2016; 56: S76–84. https://doi.org/10.1111/trf.13337
Reddoch KM, Pidcoke HF, Montgomery RK, et al. Hemostatic function of apheresis platelets stored at 4°C and 22°C. Shock 2014; 41: 54–61. https://doi.org/10.1097/shk.0000000000000082
Shea SM, Spinella PC, Thomas KA. Cold-stored platelet function is not significantly altered by agitation or manual mixing. Transfusion 2022; 62: 1850–9. https://doi.org/10.1111/trf.17005
Montgomery RK, Reddoch KM, Evani SJ, Cap AP, Ramasubramanian AK. Enhanced shear-induced platelet aggregation due to low-temperature storage. Transfusion 2013; 53: 1520–30. https://doi.org/10.1111/j.1537-2995.2012.03917.x
Nair PM, Pidcoke HF, Cap AP, Ramasubramanian AK. Effect of cold storage on shear-induced platelet aggregation and clot strength. J Trauma Acute Care Surg 2014; 77: S88–93. https://doi.org/10.1097/ta.0000000000000327
Nair PM, Meledeo MA, Wells AR, et al. Cold-stored platelets have better preserved contractile function in comparison with room temperature-stored platelets over 21 days. Transfusion 2021; 61: S68–79. https://doi.org/10.1111/trf.16530
Nair PM, Pandya SG, Dallo SF, et al. Platelets stored at 4°C contribute to superior clot properties compared to current standard-of-care through fibrin-crosslinking. Br J Haematol 2017; 178: 119–29. https://doi.org/10.1111/bjh.14751
Stolla M, Fitzpatrick L, Gettinger I, et al. In vivo viability of extended 4°C-stored autologous apheresis platelets. Transfusion 2018; 58: 2407–13. https://doi.org/10.1111/trf.14833
D'Alessandro A, Thomas KA, Stefanoni D, et al. Metabolic phenotypes of standard and cold-stored platelets. Transfusion 2020; 60: S96–106. https://doi.org/10.1111/trf.15651
Zhao HW, Serrano K, Stefanoni D, D'Alessandro A, Devine DV. In vitro characterization and metabolomic analysis of cold-stored platelets. J Proteome Res 2021; 20: 2251–65. https://doi.org/10.1021/acs.jproteome.0c00792
Kacker S, Bloch EM, Ness PM, et al. Financial impact of alternative approaches to reduce bacterial contamination of platelet transfusions. Transfusion 2019; 59: 1291–9. https://doi.org/10.1111/trf.15139
Khan RA, Syring RL. the fate of bacteria introduced into whole blood from which platelet concentrates were prepared and stored at 22 or 4 C. Transfusion 1975; 15: 363–7. https://doi.org/10.1046/j.1537-2995.1975.15476034560.x
Magron A, Laugier J, Provost P, Boilard E. Pathogen reduction technologies: the pros and cons for platelet transfusion. Platelets 2018; 29: 2–8. https://doi.org/10.1080/09537104.2017.1306046
Levy JH, Neal MD, Herman JH. Bacterial contamination of platelets for transfusion: strategies for prevention. Crit Care 2018; 22: 271. https://doi.org/10.1186/s13054-018-2212-9
Ketter PM, Kamucheka R, Arulanandam B, Akers K, Cap AP. Platelet enhancement of bacterial growth during room temperature storage: mitigation through refrigeration. Transfusion 2019; 59: 1479–89. https://doi.org/10.1111/trf.15255
Schubert P, Johnson L, Marks DC, Devine DV. Ultraviolet-based pathogen inactivation systems: untangling the molecular targets activated in platelets. Front Med (Lausanne) 2018; 5: 129. https://doi.org/10.3389/fmed.2018.00129
Devine DV. Pathogen inactivation strategies to improve blood safety: let’s not throw pathogen-reduced platelets out with their bath water. JAMA Oncol 2018; 4: 458–9. https://doi.org/10.1001/jamaoncol.2017.4949
Prudent M, D'Alessandro A, Cazenave JP, et al. Proteome changes in platelets after pathogen inactivation—an interlaboratory consensus. Transfus Med Rev 2014; 28: 72–83. https://doi.org/10.1016/j.tmrv.2014.02.002
Alter HJ, Stramer SL, Dodd RY. Emerging infectious diseases that threaten the blood supply. Semin Hematol 2007; 44: 32–41. https://doi.org/10.1053/j.seminhematol.2006.09.016
Blake JT, McTaggart K, Couture C. Estimating the impact on the inventory of implementing pathogen-reduced platelets in Canada. Transfusion 2021; 61: 3150–60. https://doi.org/10.1111/trf.16691
Arbaeen AF, Schubert P, Serrano K, Carter CJ, Culibrk B, Devine DV. Pathogen inactivation treatment of plasma and platelet concentrates and their predicted functionality in massive transfusion protocols. Transfusion 2017; 57: 1208–17. https://doi.org/10.1111/trf.14043
Pidcoke HF, McFaul SJ, Ramasubramanian AK, et al. Primary hemostatic capacity of whole blood: a comprehensive analysis of pathogen reduction and refrigeration effects over time. Transfusion 2013; 53: 137S–49.
Johnson L, Cameron M, Waters L, Padula MP, Marks DC. The impact of refrigerated storage of UVC pathogen inactivated platelet concentrates on in vitro platelet quality parameters. Vox Sang 2019; 114: 47–56. https://doi.org/10.1111/vox.12730
Agey A, Reddoch-Cardenas K, McIntosh C, et al. Effects of Intercept pathogen reduction treatment on extended cold storage of apheresis platelets. Transfusion 2021; 61: 167–77. https://doi.org/10.1111/trf.16096
Reddoch-Cardenas KM, Peltier GC, Chance TC, et al. Cold storage of platelets in platelet additive solution maintains mitochondrial integrity by limiting initiation of apoptosis-mediated pathways. Transfusion 2021; 61: 178–90. https://doi.org/10.1111/trf.16185
Wood B, Johnson L, Hyland RA, Marks DC. Maximising platelet availability by delaying cold storage. Vox Sang 2018; 113: 403–11. https://doi.org/10.1111/vox.12649
Brown BL, Wagner SJ, Hapip CA, et al. Time from apheresis platelet donation to cold storage: evaluation of platelet quality and bacterial growth. Transfusion 2022; 62: 439–47. https://doi.org/10.1111/trf.16785
Ranucci M. Hemostatic and thrombotic issues in cardiac surgery. Semin Thromb Hemost 2015; 41: 84–90. https://doi.org/10.1055/s-0034-1398383
Höfer J, Fries D, Solomon C, Velik-Salchner C, Ausserer J. A snapshot of coagulopathy after cardiopulmonary bypass. Clin Appl Thromb Hemost 2016; 22: 505–11. https://doi.org/10.1177/1076029616651146
Zhou X, Fraser CD 3rd, Suarez-Pierre A, et al. Variation in platelet transfusion practices in cardiac surgery. Innovations (Phila) 2019; 14: 134–43. https://doi.org/10.1177/1556984519836839
Karkouti K, Callum J, Wijeysundera DN, et al. Point-of-care hemostatic testing in cardiac surgery: a stepped-wedge clustered randomized controlled trial. Circulation 2016; 134: 1152–62. https://doi.org/10.1161/circulationaha.116.023956
Van Poucke S, Stevens K, Wetzels R, et al. Early platelet recovery following cardiac surgery with cardiopulmonary bypass. Platelets 2016; 27: 751–7. https://doi.org/10.3109/09537104.2016.1173665
Stubbs JR, Tran SA, Emery RL, et al. Cold platelets for trauma-associated bleeding: regulatory approval, accreditation approval, and practice implementation-just the “tip of the iceberg.” Transfusion 2017; 57: 2836–44. https://doi.org/10.1111/trf.14303
Taylor AL, Corley JB, Cap AP, et al. The U.S. Armed Services Blood Program support to U.S. Central Command 2014-2021: transformation of combat trauma resuscitation through blood product innovation and expansion of blood availability far forward. Transfusion 2022; 62: S167–76. https://doi.org/10.1111/trf.16951
Bjerkvig C, Sivertsen J, Braathen H, et al. Cold-stored whole blood in a Norwegian emergency helicopter service: an observational study on storage conditions and product quality. Transfusion 2020; 60: 1544–51. https://doi.org/10.1111/trf.15802
South Texas Blood & Tissue Center. FDA grants South Texas Blood & Tissue Center first license for new process that triples shelf life of critically needed platelets. Available from URL: https://www.globenewswire.com/en/news-release/2020/02/28/1992715/0/en/FDA-grants-South-Texas-Blood-Tissue-Center-first-license-for-new-process-that-triples-shelf-life-of-critically-needed-platelets.html (accessed May 2023).
Puri RN, Colman RW. ADP-induced platelet activation. Crit Rev Biochem Mol Biol 1997; 32: 437–502. https://doi.org/10.3109/10409239709082000
Reddoch-Cardenas KM, Bynum JA, Meledeo MA, et al. Cold-stored platelets: a product with function optimized for hemorrhage control. Transfus Apher Sci 2019; 58: 16–22. https://doi.org/10.1016/j.transci.2018.12.012
Miles J, Bailey SL, Obenaus AM, et al. Storage temperature determines platelet GPVI levels and function in mice and humans. Blood Adv 2021; 5: 3839–49. https://doi.org/10.1182/bloodadvances.2021004692
Stolla M, Bailey SL, Fang L, et al. Effects of storage time prolongation on in vivo and in vitro characteristics of 4°C–stored platelets. Transfusion 2020; 60: 613–21. https://doi.org/10.1111/trf.15669
Author contributions
Justin Lu contributed to the conception of the study, data collection, and writing the first draft of the manuscript. Keyvan Karkouti and Jeannie Callum contributed to the conception of the study, interpretation of data, and revision of manuscript drafts. Miki Peer, Philip C. Spinella, Torunn Apelseth, Thomas G. Scorer, Walter H. A. Kahr, Mark McVey, Vivek Rao, Lusine Abrahamyan, Lani Lieberman, Holly Mewhort, and Dana Devine contributed to the interpretation of data and revision of manuscript drafts. Marina Englesakis contributed to the conception, design, and execution of database search strategies, management and curation of database results, and review of manuscript drafts. Justyna Bartoszko contributed to the conception of the study, data collection, writing the first draft of manuscript, study design and analysis, and revision of manuscript drafts. All authors contributed to manuscript concept, content, and writing.
Disclosures
Justyna Bartoszko is in part supported by a merit award from the Department of Anesthesiology and Pain Medicine, University of Toronto and has received honoraria from Octapharma AG (Lachen, Switzerland). Keyvan Karkouti is in part supported by a merit award from the Department of Anesthesiology and Pain Medicine, University of Toronto. He has received research support, honoraria, or consultancy for speaking engagements from Octapharma AG, Instrumentation Laboratory Co. (Bedford, MA, USA), and Bayer AG (Leverkusen, Germany). Jeannie Callum has received research support from Canadian Blood Services and Octapharma AG. Philip C. Spinella is a consultant for Secure Transfusion Solutions (San Francisco, CA, USA).
Funding statement
No sources of funding are declared.
Disclaimer
The opinions or assertations contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Norwegian Armed Forces Medical Services.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Deputy Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, J., Karkouti, K., Peer, M. et al. Cold-stored platelets for acute bleeding in cardiac surgical patients: a narrative review. Can J Anesth/J Can Anesth 70, 1682–1700 (2023). https://doi.org/10.1007/s12630-023-02561-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02561-9