Abstract
Purpose
Intranasal insulin administration may improve cognitive function in patients with dementia and may prevent cognitive problems after surgery. Although the metabolic effects of intranasal insulin in non-surgical patients have been studied, its influence on glucose concentration during surgery is unknown.
Methods
We conducted a randomized, double-blind, placebo-contolled trial in patients scheduled for elective cardiac surgery. Patients with type 2 diabetes mellitus (T2DM) and non-T2DM patients were randomly allocated to one of three groups (normal saline, 40 international units [IU] of intranasal insulin, and 80 IU intranasal insulin). Insulin was given after the induction of general anesthesia. Glucose and plasma insulin concentrations were measured in ten-minute intervals during the first hour and every 30 min thereafter. The primary outcome was the change in glucose concentration 30 min after intranasal insulin administration.
Results
A total of 115 patients were studied, 43 of whom had T2DM. In non-T2DM patients, 40 IU intranasal insulin did not affect glucose concentration, while 80 IU intranasal insulin led to a statistically significant but not clinically important decrease in blood glucose levels (mean difference, 0.4 mMol·L−1; 95% confidence interval, 0.1 to 0.7). In T2DM patients, neither 40 IU nor 80 IU of insulin affected glucose concentration. No hypoglycemia (< 4.0 mMol·L−1) was observed after intranasal insulin administration in any patients. In non-T2DM patients, changes in plasma insulin were similar in the three groups. In T2DM patients, there was an increase in plasma insulin concentrations ten minutes after administration of 80 IU of intranasal insulin compared with saline.
Conclusions
In patients with and without T2DM undergoing elective cardiac surgery, intranasal insulin administration at doses as high as 80 IU did not cause clinically important hypoglycemia.
Trial registration
www.ClinicalTrials.gov (NCT02729064); registered 5 April 2016.
Résumé
Objectif
L’administration intranasale d’insuline pourrait améliorer la fonction cognitive des patients souffrant de démence et pourrait prévenir les problèmes cognitifs après une chirurgie. Bien que les effets métaboliques de l’insuline intranasale chez les patients non chirurgicaux aient été étudiés, son influence sur la glycémie pendant une chirurgie est inconnue.
Méthode
Nous avons réalisé une étude randomisée, à double insu, contrôlée par placebo auprès de patients devant subir une chirurgie cardiaque non urgente. Des patients atteints de diabète de type 2 et des patients non diabétiques ont été randomisés dans l’un de trois groupes (solution physiologique salée, 40 unités internationales [UI] d’insuline intranasale et 80 UI d’insuline intranasale). La solution intranasale a été administrée après l’induction de l’anesthésie générale. Les concentrations de glucose et d’insuline plasmatique ont été mesurées à des intervalles de dix minutes pendant la première heure et toutes les 30 minutes par la suite. Le critère d’évaluation principal était le changement de glycémie 30 min après l’administration intranasale d’insuline.
Résultats
Un total de 115 patients ont été étudiés, dont 43 souffraient de diabète de type 2. Chez les patients non diabétiques, 40 UI d’insuline intranasale n’ont pas affecté la glycémie, alors que 80 UI d’insuline intranasale ont entraîné une réduction statistiquement significative mais non cliniquement importante de la glycémie (différence moyenne, 0,4 mMol·L−1; intervalle de confiance de 95 %, 0,1 à 0,7). Chez les patients diabétiques, ni 40 UI ni 80 UI d’insuline n’ont affecté la glycémie. Aucune hypoglycémie (< 4,0 mMol·L−1) n’a été observée après administration intranasale d’insuline chez les patients diabétiques ou non diabétiques. Chez les patients non diabétiques, les changements de l’insuline plasmatique étaient semblables dans les trois groupes. Chez les patients diabétiques, une augmentation des concentrations d’insuline plasmatique a été observée dix minutes après l’administration de 80 UI d’insuline intranasale comparée à la solution saline.
Conclusion
Chez les patients diabétiques et non diabétiques subissant une chirurgie cardiaque non urgente, l’administration intranasale d’insuline à des doses allant jusqu’à 80 UI n’a pas causé d’hypoglycémie cliniquement importante.
Enregistrement de l’étude
www.ClinicalTrials.gov (NCT02729064); enregistrée le 5 avril 2016.
Similar content being viewed by others
Postoperative cognitive disturbances including postoperative delirium (POD) and postoperative cognitive dysfunction (POCD) are a common problem after cardiac surgery, particularly in the elderly population.1,2 So far, no effective therapy is available to treat or prevent these conditions, which have a significant impact on hospital resource utilization and quality of life.
We previously reported that insulin administration aimed at normoglycemia during cardiac surgery may prevent short- and long-term memory function postoperatively.3 While high-dose intravenous insulin therapy is labour intensive and possibly detrimental in surgical patients, recent data indicate that insulin can also be effectively and safely administered via the nasal route.4,5 Intranasal insulin administration has been shown to improve both memory performance and brain integrity in patients with Alzheimer’s disease (AD) or mild to moderate cognitive impairment.6,7,8 Furthermore, intranasal insulin may overcome compromised insulin sensitivity in the brain and decrease Tau protein and amyloid beta neurotoxicity.6,7,8
Because POCD and AD may share similar mechanisms and certain biomarkers,9 intranasal insulin may be a potential therapeutic option for POD or POCD.
Although the metabolic effects of intranasal insulin in non-surgical patients have been studied,4,10 its influence on glucose concentration in the context of the surgical stress response is unknown. Hence, this study was designed to examine the effect of intranasal insulin administered at 40 and 80 international units (IU) on circulating glucose and insulin concentrations during and after elective cardiac surgery.
Methods
Ethical consideration
This study was approved by the MUHC research ethics board (16-012-MUHC / 2017-2477, eReviews_5381) on September 1, 2016, Health Canada (# 215336) on August 24, 2016, and the Health Canada amendment (#1319430) on May 4, 2018. Written informed consent was obtained from all patients participating in the trial. The trial was registered prior to first patient enrollment at ClinicalTrials.gov (NCT02729064). The project registered at ClinicalTrials.gov consisted of two distinct studies in two different patient populations. The first study is reported here. The other study (not reported here) is in patients undergoing endovascular thoracic aneurysm repair and receiving cerebrospinal fluid (CSF) drains. It was designed to test the hypothesis that 40 and 80 IU intranasal insulin would increase the CSF level of insulin 30 min after administration. This study is still ongoing and results will be published separately.
Setting
This was a randomized, double-blind, placebo-controlled trial in patients undergoing elective cardiac procedures requiring cardiopulmonary bypass (CPB) at the Royal Victoria Hospital, a teaching hospital affiliated with McGill University and the McGill University Health Centre (MUHC), Montreal, Québec, Canada. This manuscript adheres to the Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Study population
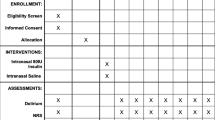
We approached patients scheduled for elective cardiac surgery between September 2016 and March 2018. The inclusion criteria were age 18 yr and older and the ability to give written informed consent. We excluded patients scheduled for off-pump coronary artery bypass grafting and requiring deep hypothermic circulatory arrest11,12 as well as patients with insulin allergy, preoperative blood glucose levels < 3.9 mMol·L−1, type-1 diabetes mellitus (T1DM), acromegaly, Cushing’s syndrome, uncontrolled hyperthyroidism, pheochromocytoma, and renal impairment. Use of insulin, glucagon like peptide-1 agonists, steroids, epinephrine, norepinephrine, and intravenous fluids containing glucose during the pre-CPB period was also an exclusion criterion.
Random allocation
Patients were classified as having or not having type 2 diabetes mellitus (T2DM) and then randomly allocated in blocks of nine to one of three groups: intranasal normal saline; 40 IU intranasal insulin; and 80 IU intranasal insulin. We executed and implemented the random allocation by labelling within each block three cards with “saline”, three with “40”, and three with “80”. We then put all cards into an envelope. Before surgery, a researcher who did not participate in patient care took out a card from the envelope, prepared the respective study drug in an unlabelled spray bottle, and administered it. Another research assistant collected the blood samples. The attending anesthesiologists and the patient were blinded to the insulin dose. The first 45 non-diabetic patients were randomized separately to allow for subsequent power calculation as described in the statistical analysis section.
In patients with T2DM, the administration of oral hypoglycemic drugs was discontinued 12 hr before surgery.
Anesthetic care and perioperative glucose management
Standard monitors13 were applied in addition to the insertion of an intra-arterial catheter, a pulmonary artery catheter and transesophageal echocardiography probe.
Patients received standardized general anesthesia using intravenous sufentanil, midazolam, and sevoflurane (end-expiratory concentration 1-2%). Muscle paralysis was established and maintained with intravenous rocuronium.
Normal saline solution was infused at a rate of 1.5 mL·kg−1·hr−1. Artificial colloid was also used to treat low filling pressures. Before CPB, heparin 400 IU·kg−1 was administered intravenously followed by additional doses, if necessary, to maintain an activating clotting time > 480 sec. Protamine was administered on a 1:1 ratio after complete separation from CPB.
Cardiopulmonary bypass was conducted with a roller pump and membrane oxygenator. During CPB, pump flow was set at 2.4 L·min−1·m−2 and mean arterial pressure maintained at 50-70 mmHg. Body temperature was kept at 32-36°C during CPB. Cardioplegia solution was free of glucose and consisted of high dose (100 mMol·L−1) potassium, which was administered for the induction and maintenance of the cardiac arrest.
Epinephrine or norepinephrine (both at 1-10 mg·kg−1·hr−1) were used as inotropes and vasopressors to maintain a systolic blood pressure at 100 mmHg before and after CPB as needed. Packed red blood cells were administered to maintain a hematocrit > 25%.
Hypoglycemia, i.e., blood glucose < 4.0 mMol·L−1, was treated with 20% glucose intravenously until normoglycemia was established. For hyperglycemia, i.e., a blood glucose >10.0 mMol·L−1, insulin was infused intravenously to maintain glycemia between 4.0 and 10.0 mMol·L−1.
Glycemic control in the intensive care unit (ICU) was as follows. Blood glucose was measured every one to two hours until the target range 4.0-10.0 mMol·L−1 was achieved, and then every four to six hours thereafter. If the blood glucose was > 10.0 mMol·L−1, an insulin infusion of 2 U·hr−1 was started and titrated according to a sliding scale based on the measured blood glucose as follows:
> 10.0 mMol·L−1, increase insulin infusion by 2 U·hr−1;
8.1-10.0 mMo·L−1, maintain current insulin infusion rate;
4.0-8.0 mMol·L−1, stop insulin infusion;
< 4.0 mMol·L−1, stop insulin infusion and administer 10 mL of 20% dextrose.
Sample collection and measurement
All samples were drawn from arterial blood. Baseline blood glucose and plasma insulin were measured. Subsequently, patients received either intranasal placebo (0.8 mL normal saline), 40 IU insulin (0.4 mL undiluted insulin and 0.4 mL saline), or 80 IU insulin (0.8 mL undiluted insulin) (Humulin R 100 IU·mL−1) via a metered nasal dispenser (Pharmasystems, Markham, ON, Canada). Arterial blood samples (2 mL) were collected at ten-minute intervals during the first 60 min following drug administration and then at 30-min intervals until the end of the surgery.
Glucose levels were measured using the StatStrip Xpress® glucose meter (Nova Biomedical, Waltham, MA, USA) immediately after blood sampling. Plasma insulin was measured using a luminescence enzyme immunoassay (Ultrasensitive Insulin Elisa, Alpco, Salem NH, USA).
In the ICU, plasma glucose concentrations were measured by the insitution’s clinical chemistry department.
Assessment of postoperative delirium and length of ICU stay
We retrospectively, by chart review, obtained data on whether or not postoperative delirium (POD) occurred in the ICU. The data abstractor was not blinded. At our institution, POD is routinely assessed and recorded by the clinical care team using the Confusion Assessment Method for the ICU (CAM-ICU)14 every eight hours (except if deeply sedated or comatose). The incidence of delirium was defined as the proportion of patients with at least one recorded episode of delirium during their ICU stay. We also obtained data on length of ICU stay.
Outcomes
The primary outcome of the study was change in blood glucose level 30 min following insulin administration in patients without T2DM, as 30 min was the previously reported time of the peak effect of intranasal insulin.12,15
Secondary outcomes were the incidence of hypoglycemia (< 4.0 mMol·L−1) during surgery and within the first 24 hr after surgery, change in plasma insulin concentration during surgery, and incidence of POD in the ICU.
Severe hyperglycemia was defined as a blood glucose level > 10.0 mMol·L−1, mild hyperglycemia was defined as a blood glucose level 6.1-10.0 mMol·L−1, normoglycemia was defined as a blood glucose level 4.0-6.0 mMol·L−1, mild hypoglycemia was defined as a blood glucose level 2.2-3.9 mMol·L−1, and severe hypoglycemia was defined as a blood glucose level < 2.2 mMol·L−1.
Sample size
Power calculation determined that 22 patients without T2DM were required per group to detect a blood glucose difference of 0.5 mMol·L−1 between groups at 30 min after intranasal insulin administration, assuming a standard deviation (SD) of 0.5 mMol·L−1 in each group, an alpha level of 0.05, and a power of 0.8 (one-way analysis of variance).
Power calculation was based on previous studies showing small (0.2-0.5 mMol·L−1, SD 0.5 mMol·L−1) but significant decreases in blood glucose 30 min following intranasal insulin (40 IU and 80 IU) in non-surgical subjects16,17 and on data obtained in the first 45 non-diabetic patients of this study showing similar mean (SD) and changes in glycemia (saline: 0.0 [0.5] mMol·L−1; 40 IU insulin: 0.2 [0.5] mMol·L−1; 80 IU insulin: 0.5 [0.5] mMol·L−1).
Considering a block size of nine, we had planned to recruit 24 patients per group.
Statistical analysis
Patient characteristics are presented as mean (SD) or median [interquartile range (IQR)] for continuous variables, and counts (percentages) for categorical variables. A pre-specified two-way analysis of variance detected a statistically significant difference between diabetics and non-diabetics; therefore, the changes in blood glucose were analyzed by strata of diabetes/non-diabetes. Changes in blood glucose and insulin levels between groups were then analyzed by Student’s t tests and adjusted using the Bonferroni method. Length of ICU stay was analyzed by the Kruskal-Wallis test. The Chi square test was used to analyze POD incidence. We performed all statistical analyses using SAS version 9.4 (SAS Institute, Cary, NC, USA). All reported P values were two-sided, and P < 0.05 was considered significant.
Results
Study population
We analyzed 115 patients, 43 (36%) of whom had T2DM (Fig. 1). Patients’ characteristics are presented in Table 1. A total of 1,106 blood glucose measurements (average 9.6 per patient) during surgery were recorded. We also collected results of 548 measurements in the ICU during the first 24 postoperative hours.
Primary and secondary endpoints
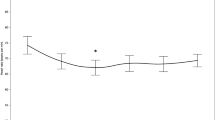
While 40 IU of insulin did not affect glycemia in non-diabetic patients 30 min after insulin administration (mean difference, 0.3 mMol·L−1; 95% confidence interval [CI], -0.1 to 0.6; P = 0.07), 80 IU of insulin slightly, but significantly, decreased glucose concentration 30, 50, and 60 min after insulin administration (mean difference 30 min after insulin administration, 0.4 mMol·L−1; 95% CI, 0.1 to 0.7; P < 0.001, Fig. 2A).
A Blood glucose concentrations in patients without type 2 diabetes. Error bars represent standard deviation of the delta blood glucose concentration. *P < 0.05 vs saline group. B Blood glucose concentrations in patients with type 2 diabetes. Error bars represent standard deviation of the delta blood glucose concentration. *P < 0.05 vs saline group
In patients with T2DM, neither 40 IU nor 80 IU of insulin had a significant effect on blood glucose 30 min after administration (saline vs 40 IU: mean difference, 0.5 mMol·L−1; 95% CI, -0.3 to 1.3; P = 0.18; saline vs 80 IU: mean difference, 0.3 mMol·L−1; 95% CI, -0.4 to 1.0; P = 0.38). Patients with T2DM receiving 40 IU insulin showed significantly lower glucose levels 180 min after insulin administration than patients receiving saline did (saline vs 40 IU: mean difference, 2.1 mMol·L−1; 95% CI, 0.9 to 3.4; P = 0.004; saline vs 80 IU: 0.0 mMol·L−1; 95% CI, 2.7 to -2.6; P = 0.99). At 180 min after insulin administration, some patients were excluded because intravenous insulin was administered to treat hyperglycemia.
No patient receiving insulin experienced hypoglycemia. One patient in the saline group experienced one episode of mild hypoglycemia in the ICU (Table 2).
Exploratory linear mixed-effect models showed that T2DM affected the change in blood glucose (F = 6.0, P < 0.001) without any impact on plasma insulin changes (F = 1.3, P = 0.22).
In non-diabetic patients, changes in plasma insulin were similar in the three groups (P = 0.08, Fig. 3A). Patients with T2DM who received 80 IU insulin showed increased plasma insulin concentrations ten minutes after administration compared with patients who received saline (P < 0.001, Fig. 3B).
A Plasma insulin concentrations in patients without type 2 diabetes. Error bars represent standard deviation of the delta plasma insulin concentration. B Plasma insulin concentrations in patients with type 2 diabetes. Error bars represent standard deviation of the delta plasma insulin concentration. *P < 0.05 vs saline group
Eleven patients (30%) in the saline group, seven patients (18%) in the low-dose insulin group, and six patients (15%) in the high-dose insulin group had a least one episode of POD in the ICU (P = 0.69). Median [IQR] length of ICU stay was similar between the three groups (saline group: 49 [45-69] hr, 40 IU insulin group: 46 [44-68] hr, and 80 IU insulin group: 49 [43-92] hr; P = 0.57). Length of ICU stay was longer in patients with POD (77 [45-69] hr) than in those without POD (46 [44 to 69] hr) (P < 0.001).
Discussion
The results of the present study show that intranasal insulin administration, even at larger doses (80 IU), in non-diabetic and diabetic patients did not cause clinically important hypoglycemia during and after open heart surgery.
Cognitive problems in patients after cardiac surgery18,19 can be detected in up to 50% of elderly patients and are associated with poor long-term outcomes.19,20,21 We have previously shown that intravenous administration of large doses of insulin together with glucose to maintain normoglycemia intraoperatively is associated with improved short- and long-term memory function after cardiac surgery.3 However, this protocol (called Glucose and Insulin administration while maintaining Normoglycemia) is labour intensive and bears the potential risk of hypoglycemia.22
Spraying insulin into the nasal cavity is a relatively novel alternative approach to administering insulin in humans. Studies in normal subjects showed that intranasally administered insulin, bypassing the blood-brain barrier (BBB), leads to sustained elevation of insulin concentrations in the CSF without spilling over into the circulation and exerting metabolic effects systemically.5 Patients with AD or cognitive impairment repeatedly receiving 40 IU of intranasal insulin showed significant improvement of memory performance and brain integrity.6,7,8,23,24
While the mechanisms underlying POCD are not fully understood, some of its pathophysiologic features are similar to those observed in subjects with neurodegenerative disorders.25 Patients with AD are known to have decreased CSF insulin levels and impaired brain insulin sensitivity,7,26,27,28 which has been identified as an important pathogenetic factor and a target for pharmacologic interventions.29 More recently, insulin resistance has been shown to contribute to POCD after cardiac valve surgery.30
Studies in normal subjects indicate that CSF insulin levels increase within seven minutes of intranasal insulin administration reaching a peak after 30 min and remaining elevated for more than 80 min.5 A small spillover of 1-2%12 into the peripheral circulation was observed. Intranasal administration of 25 IU insulin was accompanied by an increase in plasma insulin concentrations up to 25 μIU·mL−1 after 10-20 min. Furthermore, the circulating blood glucose concentration decreased by 0.5 mMol·L−1 after 40 min, which returned to baseline after 90-210 min.12,15,31 In the present study in cardiac surgery patients, intranasal administration of insulin at doses of 40 IU and 80 IU did not cause hypoglycemia intraoperatively whereas 80 IU insulin, similar to observations in normal subjects, slightly decreased blood glucose levels 30-60 min after administration. Insulin spillover into the systemic circulation still appeared to occur, particularly in diabetic patients receiving 80 IU (~5 μU·mL−1 increase), but to a much smaller extent than in the non-surgical setting. This discrepancy may be explained by increased BBB permeability and cerebral insulin uptake during general anesthesia as seen in older animals.32
In the ICU, during the first postoperative 24 hr, only one non-diabetic patient in the saline group experienced a hypoglycemic episode. Taking into account that up to 11% of surgical patients have been reported to develop hypoglycemia (< 3.3 or 3.9 mMol·L−1) when treated with sliding scales aimed at maintaining glucose concentration between 6.1 and 10.0 mMol·L−1,33,34,35,36 we conclude that intranasal administration of 40 IU and 80 IU insulin does not cause hypoglycemia perioperatively. In fact, in 21% of the samples drawn in the present study, glucose levels exceeded 10.0 mMol·L−1 even in the absence of T2DM.
Patients receiving intranasal insulin did not show a statistically significantly lower POD incidence than patients in the saline group did. Larger, prospective studies are needed to demonstrate whether this potential effect is clinically important.
We acknowledge several limitations of the present study. Firstly, because CSF insulin levels could not be measured in our patients, it remains unknown whether insulin was actually taken up by the brain. Nevertheless, intranasal insulin dispensers similar to the ones used in the present protocol were shown to be effective in previous studies.5
Secondly, no attempts have been made to quantify the magnitude of the stress response to surgery. It is therefore unknown if the plasma concentrations of counterregulatory hormones such as cortisol and catecholamines were comparable in the three study groups. Nevertheless, anesthesia care was standardized for all patients and catecholamine use as well as hemodynamics were similar in all groups, making any significant influence of endocrine alterations unlikely.
Thirdly, POD data were obtained retrospectively in a small number of patients. In addition, POD was only assessed in the ICU, and ICU stay in patients with POD was longer than in patients without POD. Hence, the true incidence of POD during the entire postoperative course may have been underestimated. A much larger study population (at least 280 patients in each group) would be needed to detect a statistically significant impact of 40 IU intranasal insulin on POD (alpha = 0.05, power = 0.9).
In summary, insulin intranasally administered before cardiac surgery, even at large doses up to 80 IU, was not associated with significant hypoglycemia. It therefore appears to be metabolically safe to study the potentially neuroprotective effects of intranasal insulin in cardiac surgery patients.
References
Mashour GA, Woodrum DT, Avidan MS. Neurological complications of surgery and anaesthesia. Br J Anaesth 2015; 114: 194-203.
Arora SS, Gooch JL, Garcia PS. Postoperative cognitive dysfunction, Alzheimer’s disease, and anesthesia. Int J Neurosci 2014; 124: 236-42.
Schricker T, Sato H, Beaudry T, Codere T, Hatzakorzian R, Pruessner JC. Intraoperative maintenance of normoglycemia with insulin and glucose preserves verbal learning after cardiac surgery. PLoS One 2014; DOI:https://doi.org/10.1371/journal.pone.0099661.
Heni M, Kullmann S, Ketterer C, et al. Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward-related human brain regions. Diabetologia 2012; 55: 1773-82.
Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 2002; 5: 514-6.
Reger MA, Watson GS, Frey WH 2nd, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging 2006; 27: 451-8.
Freiherr J, Hallschmid M, Frey WH 2nd, et al. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs 2013; 27: 505-14.
Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 2008; 70: 440-8.
Bittner EA, Yue Y, Xie Z. Brief review: anesthetic neurotoxicity in the elderly, cognitive dysfunction and Alzheimer’s disease. Can J Anesth 2011; 58: 216-23.
Guthoff M, Grichisch Y, Canova C, et al. Insulin modulates food-related activity in the central nervous system. J Clin Endocrinol Metab 2010; 95: 748-55.
Thiessen S, Vanhorebeek I, Van den Berghe G. Glycemic control and outcome related to cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol 2015; 29: 177-87.
Leary AC, Stote RM, Breedt HJ, O’Brien J, Buckley B. Pharmacokinetics and pharmacodynamics of intranasal insulin administered to healthy subjects in escalating doses. Diabetes Technol Ther 2005; 7: 124-30.
Dobson G, Chong M, Chow L, et al. Guidelines to the practice of anesthesia - revised edition 2017. Can J Anesth 2017; 64: 65-91.
Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med 2001; 29: 1370-9.
Newman SP, Steed KP, Hardy JG, Wilding IR, Hooper G, Sparrow RA. The distribution of an intranasal insulin formulation in healthy volunteers: effect of different administration techniques. J Pharm Pharmacol 1994; 46: 657-60.
Brünner YF, Benedict C, Freiherr J. Intranasal insulin reduces olfactory sensitivity in normosmic humans. J Clin Endocrinol Metab 2013; 98: E1626-30.
Feld GB, Wilhem I, Benedict C, et al. Central nervous insulin signaling in sleep-associated memory formation and neuroendocrine regulation. Neuropsychopharmacology 2016; 41: 1540-50.
Ahlgren E, Lundqvist A, Nordlund A, Aren C, Rutberg H. Neurocognitive impairment and driving performance after coronary artery bypass surgery. Eur J Cardiothorac Surg 2003; 23: 334-40.
O’Brien H, Mohan H, O’Hare C, Reynolds JV, Kenny RA. Mind over matter? The hidden epidemic of cognitive dysfunction in the older surgical patient. Ann Surg 2017; 265: 677-91.
Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008; 108: 18-30.
Steinmetz J, Siersma V, Kessing LV, Rasmussen LS; ISPOCD Group. Is postoperative cognitive dysfunction a risk factor for dementia? A cohort follow-up study. Br J Anaesth 2013; 110(Suppl 1): i92-7.
Engoren M, Schwann TA, Habib RH. Hyperglycemia, hypoglycemia, and glycemic complexity are associated with worse outcomes after surgery. J Crit Care 2014; 29: 611-7.
Chen Y, Run X, Liang Z, et al. Intranasal insulin prevents anesthesia-induced hyperphosphorylation of tau in 3xTg-AD mice. Front Aging Neurosci 2014; DOI:https://doi.org/10.3389/fnagi.2014.00100.
Zhang Y, Dai C, Chen Y, Iqbal K, Liu F, Gong CX. Intranasal insulin prevents anesthesia-induced spatial learning and memory deficit in mice. Sci Rep 2016; DOI:https://doi.org/10.1038/srep21186.
Xie Z, Tanzi RE. Alzheimer’s disease and post-operative cognitive dysfunction. Exp Gerontol 2006; 41: 346-59.
Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis 2005; 8: 247-68.
Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012; 122: 1316-38.
Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D Jr. Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology 1998; 50: 164-8.
Diehl T, Mullins R, Kapogiannis D. Insulin resistance in Alzheimer’s disease. Transl Res 2017; 183: 26-40.
Tang N, Jiang R, Wang X, et al. Insulin resistance plays a potential role in postoperative cognitive dysfunction in patients following cardiac valve surgery. Brain Res 2017; 1657: 377-82.
Leary AC, Dowling M, Cussen K, O’Brien J, Stote RM. Pharmacokinetics and pharmacodynamics of intranasal insulin spray (Nasulin) administered to healthy male volunteers: infuence of the nasal cycle. J Diabetes Sci Technol 2008; 2: 1054-60.
Acharya NK, Goldwaser EL, Forsberg MM, et al. Sevoflurane and isoflurane induce structural changes in brain vascular endothelial cells and increase blood-brain barrier permeability: possible link to postoperative delirium and cognitive decline. Brain Res 2015; 1620: 29-41.
Lazar HL, McDonnell MM, Chipkin S, Fitzgerald C, Bliss C, Cabral H. Effects of aggressive versus moderate glycemic control on clinical outcomes in diabetic coronary artery bypass graft patients. Ann Surg 2011; 254: 458-63; discussion 463-4.
Desai SP, Henry LL, Holmes SD, et al. Strict versus liberal target range for perioperative glucose in patients undergoing coronary artery bypass grafting: a prospective randomized controlled trial. J Thorac Cardiovasc Surg 2012; 143: 318-25.
Mulla I, Schmidt K, Cashy J, et al. Comparison of glycemic and surgical outcomes after change in glycemic targets in cardiac surgery patients. Diabetes Care 2014; 37: 2960-5.
Umpierrez G, Cardona S, Pasquel F, et al. Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG Trial. Diabetes care 2015; 38: 1665-72.
Author contributions
Patricia Roque and Yosuke Nakadate contributed to acquisition, analysis and interpretation of data, and drafting the manuscript. Hiroaki Sato contributed to study conception and, design, analysis and interpretation of data, and drafting the manuscript. Tamaki Sato contributed to study design, collection and, analysis of data, and drafting the manuscript. Linda Wykes contributed to study conception and, design, and revising the manuscript. Akiko Kawakami contributed to acquisition of data and drafting the manuscript. Hiroshi Yokomichi contributed to analysis of data and drafting the manuscript. Takashi Matsukawa contributed to revising the manuscript. Thomas Schricker contributed to study conception and, design, interpretation of the data, and drafting and revising the manuscript.
Disclosures
All authors declare that they have no financial or non-financial interests that may be relevant to the submitted work.
Funding statement
The study was supported by a departmental fund (account number 65788), the Bourse Rosario Denis From the Association of Quebec Anesthesiologists (H. Sato, AAQ), and a salary grant from the Yamanashi Prefecture (Y. Nakadate).
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Deputy Editor-in-Chief, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roque, P., Nakadate, Y., Sato, H. et al. Intranasal administration of 40 and 80 units of insulin does not cause hypoglycemia during cardiac surgery: a randomized controlled trial. Can J Anesth/J Can Anesth 68, 991–999 (2021). https://doi.org/10.1007/s12630-021-01969-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-021-01969-5