Abstract
Purpose
Hip and knee arthroplasty surgeries are associated with embolism of materials such as air, cement, and fat. Patent foramen ovale (PFO) is a common congenital cardiac condition that has been reported to lead to paradoxical embolism. This observational study aimed to investigate if the presence of a PFO was associated with an increased risk of postoperative delirium in patients undergoing primary elective hip or knee arthroplasties.
Method
This was a prospective cohort study at a tertiary teaching hospital. We enrolled patients undergoing primary elective hip or knee arthroplasty who did not have any risk factors for embolism or delirium. Bedside transthoracic echocardiography (TTE) with a bubble study was performed on all patients to detect the presence of PFO. The primary outcome was postoperative delirium as assessed by the standardized Confusion Assessment Method. Secondary outcomes included the ease of performing a TTE bubble study in the perioperative setting, the quality of the TTE images, length of stay, major cardiovascular and neurologic complications, and effects of anesthetic or analgesic management techniques on delirium.
Results
Two hundred two patients completed the study. The median [interquartile range] duration of stay was 2 [2-3] days. Only 16 patients (8%) had a positive bubble study. Postoperative delirium was observed in only one patient. Major adverse events were not seen. The inter-rater reliability for the TTE image quality scores was fair (kappa statistic = 0.22).
Conclusion
Given the very low incidence of PFO and postoperative delirium in this study, we could not form any conclusions regarding the impact of a PFO on important outcomes including delirium or other major adverse events. No recommendation can be made regarding screening for PFO in patients scheduled for lower extremity arthroplasty surgery.
Trial registration
ClinicalTrials.gov (NCT02400892). Registered 27 March 2015.
Résumé
Objectif
Les arthroplasties de hanche et de genou sont associées à des embolies d’air, de ciment et de graisse, notamment. Le foramen ovale perméable (FOP) est une condition cardiaque congénitale fréquente qui peut entraîner une embolie paradoxale. Cette étude observationnelle a cherché à savoir si la présence d’un FOP était associée à une augmentation du risque de delirium postopératoire chez des patients subissant une arthroplastie élective primaire de hanche ou de genou.
Méthode
Il s’agissait d’une étude prospective de cohorte dans un hôpital universitaire tertiaire. Nous avons recruté des patients subissant une arthroplastie élective primaire de hanche ou de genou sans facteurs de risque d’embolie ou de delirium. Un échocardiogramme transthoracique (ETT) couplé à une épreuve de contraste avec microbulles a été réalisé chez tous les patients pour détecter la présence d’un FOP. Le principal critère d’évaluation était le delirium postopératoire évalué par la méthode standardisée d’évaluation de l’état confusionnel. Les critères d’évaluation secondaires ont inclus la facilité de réalisation d’une ETT avec épreuve de contraste et microbulles dans un cadre périopératoire, la qualité des images de l’ETT, la durée de séjour, les complications cardiovasculaires et neurologiques majeures, ainsi que les effets de l’anesthésie ou des techniques de gestion de la douleur sur le delirium.
Résultats
Deux cent deux patients ont terminé l’étude. La durée médiane de séjour (écart interquartile) a été de 2 (2 à 3) jours. Seulement 16 patients (8 %) ont eu une étude par microbulles positive. Un delirium post opératoire n’a été observé que chez un seul patient. Aucun événement indésirable majeur n’a été constaté. La fiabilité inter-évaluateur pour les scores de qualité d’image de l’ETT a été faible (coefficient kappa = 0,22).
Conclusion
Compte tenu de la très faible incidence du FOP et du delirium postopératoire dans cette étude, nous n’avons pas pu établir de conclusions concernant les répercussions du FOP sur les critères d’évaluation dont le delirium ou d’autres événements indésirables majeurs. Aucune recommandation ne peut être faite concernant le dépistage du FOP chez les patients devant subir une arthroplastie élective des membres inférieurs.
Enregistrement de l’essai clinique
ClinicalTrials.gov (NCT02400892). Enregistré le 27 mars 2015.
Similar content being viewed by others
Total joint arthroplasty is effective at treating joint pain and improving functional status, especially for osteoarthritis. In Canada, as in many developed countries, large volumes of such procedures are performed annually with a trend for ongoing increases. The most recent data from the Canadian Institute for Health Information showed a 19-23% increase in hip and knee replacements in 2013-2014.1 Postoperative delirium has often been described as a common complication following hip and knee replacement surgeries and is associated with longer hospital stays, impaired recovery and rehabilitation, and an increased risk of other complications.2,3 It has been estimated that the total annual costs attributable to delirium after hospitalization vary from $16,303 to $64,421 USD per patient.4 Efforts to minimize and prevent postoperative delirium could therefore have extensive patient outcome and economic benefits on our healthcare system.
A patent foramen ovale (PFO) is a common congenital heart defect due to incomplete fusion of the septum primum and secundum, creating a communication between the left and right atria.5 A patent foramen ovale occurs commonly in the general population, with a prevalence of 15-35% in different populations.5 It has been reported to lead to paradoxical embolization during arthroplasty procedures, although paradoxical embolism of microfat particles has also been shown to occur in the absence of a PFO.6 Cerebral embolic phenomena are known to occur during intramedullary nailing of long bones and both cemented and uncemented arthroplasty procedures.7,8,9,10,11 Paradoxical embolization has been reported through a patent foramen ovale intraoperatively with intramedullary nailing.6 It is currently still unclear whether postoperative cognitive function is affected by paradoxical embolization. Previously published literature with small sample sizes has not established its clinical significance.8,11,12
There are a variety of methods to diagnose a PFO. One non-invasive test is a transthoracic echocardiogram (TTE) with a bubble study, which has been shown to have a sensitivity of 88% and specificity of 97% compared with the transesophageal echocardiogram (TEE), which is considered to be the gold standard.13 TTE may therefore be a convenient diagnostic tool for rapid point-of-care assessment for finding a PFO. The use of TTE for different applications during the perioperative period is increasingly common.14 We therefore sought, through a prospective observational study, to examine the association of a PFO, as detected with TTE, with the incidence of postoperative delirium among patients undergoing elective hip or knee arthroplasty.
Methods
We conducted a single-centre prospective cohort study at a university hospital—a tertiary-level care center affiliated with Western University in London, Ontario, Canada. The study occurred between March 2015 and June 2016. Western University’s Institutional Research Ethics Board (REB) approval was obtained for this study in February 2015. This trial was registered at ClinicalTrials.gov (NCT02400892). All participants provided written informed consent.
The primary objective was to determine if the presence of a PFO was associated with an increased risk of developing postoperative delirium in patients undergoing primary elective hip or knee arthroplasty procedures. We hypothesized that the presence of a PFO would be associated with postoperative delirium. Secondary outcomes included assessing for the association of a PFO with length of hospital stay and major complications (myocardial infarction, stroke, heart failure, thromboembolism, arrhythmia, intensive care unit [ICU] admission, acute kidney injury and death). The quality of obtained TTE scans as well as study feasibility (the ability to complete the study without delaying the case start time) was also assessed in post-hoc analysis.
Participants, inclusion and exclusion criteria
Patients scheduled in the preadmission clinic with an appointment for hip or knee arthroplasty were identified for eligibility assessment. Inclusion criteria included adult (18 yr and older) patients planned for an elective primary hip or knee replacement procedure. Exclusion criteria included: 1) abnormalities that may confound structural cardiac assessment for a PFO (history of cardiac surgery or prosthetic heart valves, other structural heart abnormalities, pacemaker or implantable cardioverter defibrillator in situ), 2) pre-existing medical problems that would bias delirium assessments (poor English proficiency, transient ischemic attack or stroke within the past year, neurologic conditions causing ongoing functional problems, psychiatric disease causing functional impairment, significant visual or hearing impairment), 3) conditions increasing embolism risk (new diagnosis of atrial fibrillation within the past three months, deep venous thrombosis or pulmonary embolism within the past year), 4) undergoing a revision procedure, or 5) unable to provide informed consent. Eligible patients were approached in the preadmission clinic for informed consent.
Transthoracic echocardiogram bubble study
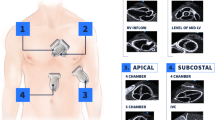
On the day of their operation, participants underwent a bedside TTE bubble study. Research team members, with more than ten years of ultrasound experience, after re-confirming appropriate consent, performed the bubble study in either the preoperative holding area or preoperative block room where patients underwent spinal and/or peripheral nerve blockade. Several patients underwent examination after surgery either in the recovery room or, rarely, on the inpatient floor. Decision as to when and where the studies were performed was made based on the flow of operating room scheduling with the goal of avoiding delays in patient care. A Philips (Koninklijke Philips N.V, Markham, ON, Canada) Sparq ultrasound system with a cardiac transducer (Phillips product code S4-2; broadband sector array transducer) was used to perform the TTE bubble studies. An adequate four-chamber view of the heart was obtained (apical, subcostal, or parasternal view). A standardized bubble study protocol was used. The patient was asked to perform a Valsalva maneuver and agitated saline was injected intravenously via a three-way stop-cock. (Agitated saline was made up of 9 mL of normal saline with 1 mL of air). The Valsalva was then released by the patient and the scan was recorded using six-second loops. Adequate bubble study images required complete opacification of the right atrium. Recorded files were stored electronically in a private drive within the hospital firewall only accessible by research team members. The files were saved in DICOM format and were viewed using the Philips DICOM Viewer (version 3.0, freeware). Files were saved using numeric study ID without other identifier or patient information. Each file was independently reviewed by two consultant cardiac anesthesiologists (research team members) at a later time. The bubble study was considered positive if any bubbles appeared in the left atrium within the first five cardiac cycles. Disagreement was resolved by consensus. A post hoc analysis was conducted to grade image quality based on a previously described scale (Table 1).15
Postoperative delirium and follow-up
The primary outcome of this study was the incidence of postoperative delirium. Patients were assessed on a daily basis starting on postoperative day 1. Their charts were reviewed for intraoperative data (type of the surgery, method of the anesthesia, and surgical duration in minutes), mode of analgesia (peripheral nerve block, use of intrathecal long acting narcotic, patient controlled intravenous narcotic analgesia, multimodal analgesia), and any postoperative complications including myocardial infarction, cerebrovascular event or venous thromboembolism, heart failure, blood transfusion, admission to intensive care unit, or death). Delirium screening was performed by bedside nurses using standardized Confusion Assessment Method (CAM) forms.16 The CAM is a widely recognized, easy-to-use diagnostic tool for delirium with a sensitivity of 94% and a specificity of 89%.17 Patients were followed daily until their day of discharge or up until postoperative day 7, whichever occurred earlier. The duration of hospital stay was also recorded.
Sample size calculation
The reported incidence of postoperative delirium in elective hip and knee arthroplasty procedures varies widely—from 9-28%.2 The incidence of PFO in the general population also varies widely—from 15-35%.5 We assumed an incidence of delirium of 10% in the control group to be conservative in our sample size calculation. We aimed to be able to detect a three-fold (incidence of 30%) or higher risk of delirium in patients with a PFO compared with those who did not have a PFO. It was thought that this increase in risk in patients with a PFO would be large enough to justify the time and effort required for routine screening because it still represents only a small absolute number of patients who develop delirium. Additionally, it was assumed that only 25% of the general population might have a PFO. The sample size required was calculated to be 168 patients, aiming for a power of 80% and confidence interval of 95%. It was difficult to predict the exact number of patients that would be required given the variable incidence of PFO in the general population. To account for this variability, and any potential attrition, a sample size of 200 subjects was used.
Statistical analysis
We separated our sample into two groups based on whether they had a PFO. Data were described using counts and percentages or medians and interquartile ranges [IQR]. Between group comparisons used Fisher’s exact test (categorical variables) or the Wilcoxon rank-sum test (continuous variables). The difference in risk of experiencing outcomes between the two groups was compared using the relative risk or the difference in medians (with the 95% confidence interval [CI] for the latter being computed using bootstrapping). No adjusted analyses were performed given the low event rates seen for all outcomes (and hence very limited statistical power for adjusted comparisons). The inter-rater agreement for the two raters of the echocardiograms was computed using the kappa coefficient. P < 0.05 was considered to be statistically significant. Stata version 14 (StataCorp LLC, College Station, TX, USA) was used for all analyses.
Results
Five hundred ninety-one patients were screened for eligibility in the Preadmission Clinic, with 245 of them being approached for recruitment. Of these, 226 patients were consented and enrolled, and 202 patients went on to complete the study (Fig. 1).
Table 2 shows the patient characteristics in our study population. A total of 16 patients (8%) had a positive bubble study for a PFO; an example is shown in Fig. 2. Both PFO-positive and -negative groups had approximately equal distribution of hip vs knee arthroplasty procedures. The majority of patients in both groups were female (58% no PFO group, 69% PFO positive group). The PFO-negative group had a higher median [IQR] body mass index (31 [28-37] kg·m−2) than the PFO-positive group (28 [26-33] kg·m−2). The median durations of surgery were similar between the two groups. A greater proportion of PFO-negative patients received a spinal anesthetic (80%) vs the PFO-positive group (69%).
The primary outcome of the study (the incidence of postoperative delirium) was recorded in a single patient (Table 3). The median [IQR] duration of hospital stay was 2 [2-3] days in both groups. There were no major adverse events including myocardial infarction, stroke, heart failure, thromboembolism, arrhythmia, ICU admission, and death.
The inter-rater reliability between our reviewers was fair with a kappa statistic of 0.22 (P < 0.001) (Table 4). For our post hoc analysis on image quality, the actual agreement on overall quality of the images was on 47% of the scans. The majority of the studies’ quality was rated excellent or good (67-72%), while only 12-16 (6-8%) were rated very poor. Post hoc analysis also showed the feasibility of routine perioperative bedside TTE to assess patients, as we found the study was completed quickly and did not affect the flow in the surgical unit. Some studies were performed after the surgical procedure; however, this was due to a lack of availability of a sonographer (anesthesiologist) and not due to time constraints.
Discussion
To our knowledge, this is the largest study to date performed to assess the risk of postoperative delirium associated with the presence of a PFO. Unfortunately, the low incidences of postoperative delirium (one patient, 6.3% of the PFO group) and PFO incidence (8%) meant that no meaningful conclusions could be drawn regarding our primary outcome. Regarding our secondary outcomes, there were very few complications and the median duration of hospital stay was two days. The focused TTE bubble study was a rapid and easy method for assessing a PFO. Given the incorporation of bedside ultrasound assessments in perioperative care today, this is a technique that could be easily taught to trainees should concern regarding the existence of a PFO arise.
The prevalence of a PFO as identified by TTE bubble studies was low in this trial. Although it has been reported that TTE has a high sensitivity (88%) and specificity (97%) compared with TEE for identifying PFOs,13 it has also been reported that TTE has a much lower sensitivity for detecting small shunts with a prevalence of 14.9%.5 Known factors for the lower accuracy of TTE with contrast injection to detect a PFO include inadequate imaging windows, PFOs with small associated shunts, older age, and the ability to properly perform a Valsalva maneuver.5,13 Our rate of PFO detection may have been much lower for a variety of reasons. A right-to-left shunt through the PFO may have been more difficult to attain in this patient population given that they were all fasted and relatively intravascularly deplete, with significant sympathetic stimulation from perioperative anxiety. It was at times difficult to obtain good quality images because of a combination of body habitus and difficulties positioning patients because of pre-existing and postoperative pain (and residual intrathecal block for scans performed postoperatively). The median body mass index of our study population was 28 and 31 in the PFO-positive and PFO-negative groups, respectively. We also found that we could not achieve complete opacification of the right atrium in all of our study patients despite multiple attempts and adequate volumes of contrast fluid and flush injected. As is usual for surgical patients at our centre, patients typically had an intravenous catheter placed by the surgical prep nurse in their hand or forearm. Ideally, the contrast injectate should be injected via an antecubital vein rather than a more peripheral vein. Transthoracic echocardiography scans were reviewed later for interpretation in dark rooms. During routine clinical application, this would not be typical as point-of-care assessments are usually made at the bedside, often in suboptimal lighting conditions. Our imaging assessment may have been more sensitive because of the conditions during image interpretation.
The question we address in this study is whether a bubble study performed using point-of-care ultrasound was of sufficient sensitivity to diagnose a PFO in enough patients that it would impact outcome. The use of transthoracic ultrasound was chosen as it is widely available in many settings in which hip and knee surgery is performed. Access to TEE or Doppler ultrasound, both superior methods to detect PFOs, may not be available. Training of anesthesiologists to use these modalities may also be limited. Finally, TEE requires sedation if patients are having regional techniques (spinal) for the surgical procedure. Despite these limitations it might not be unreasonable to assume that TTE would likely identify large PFOs, or those with an easy-to-induce right-to-left shunt, those likely to have the effect on delirium.
We performed a post-hoc review of image quality; the inter-rater reliability for the quality scores for the obtained TTE images was surprisingly low, with a kappa statistic of 0.22. The actual agreement was on only 47% of the scans looking at the score used for quality assessment. Regardless, the majority of the scans’ quality was rated excellent or good (67-72%), while only 6-8% were rated very poor. This may partly reflect the difficulty in obtaining optimal images in this patient population and the subsequent challenges with interpretation. Given the rise in popularity of point-of-care ultrasound studies performed perioperatively, this is also a reminder to avoid overzealous interpretation of suboptimal images.
The very low incidence of postoperative delirium may be partially explained by the very strict exclusion criteria used to minimize risk factors for delirium. Although we did not have age restrictions for recruitment, our study population was relatively young with median ages of 62 and 64. We thus selected a group of patients at relatively low risk for developing delirium. Although the CAM is a well-validated tool for delirium screening, the fact that they were only completed on a daily basis may miss episodes of delirium, which is known to have a fluctuating course over the day.
Given the low incidences of both PFO and delirium that we observed, to make any inferences regarding the effect of PFO on postoperative delirium would require a much larger study than ours. Future research may elect to focus on higher-risk populations with a higher expected risk of postoperative delirium, such as non-elective hip fracture fixation patients. Nevertheless, this study proved the feasibility of routine perioperative bedside TTE to assess patients; the study can be done quickly and does not affect the flow in the surgical unit.
References
Canadian Institute for Health Information. Hip and Knee Replacements in Canada: Canadian Joint Replacement Registry 2015 Annual Report. CIHI; 2015. https://secure.cihi.ca/free_products/CJRR_2015_Annual_Report_EN.pdf (accessed January 2018).
Bruce AJ, Ritchie CW, Blizard R, Lai R, Raven P. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int Psychogeriatr 2007; 19: 197-214.
Freter SH, Dunbar MJ, MacLeod H, Morrison M, MacKnight C, Rockwood K. Predicting post-operative delirium in elective orthopaedic patients: the Delirium Elderly At-Risk (DEAR) instrument. Age Ageing 2005; 34: 169-71.
Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008; 168: 27-32.
Di Tullio MR. Patent foramen ovale: echocardiographic detection and clinical relevance in stroke. J Am Soc Echocardiogr 2010; 23: 144-55.
Byrick RJ, Mullen JB, Mazer CD, Guest CB. Transpulmonary systemic fat embolism. Studies in mongrel dogs after cemented arthroplasty. Am J Respir Crit Care Med 1994; 150: 1416-22.
Christie J, Robinson CM, Pell AC, McBirnie J, Burnett R. Transcardiac echocardiography during invasive intramedullary procedures. J Bone Joint Surg Br 1995; 77: 450-5.
Rodriguez RA, Tellier A, Grabowski J, et al. Cognitive dysfunction after total knee arthroplasty: effects of intraoperative cerebral embolization and postoperative complications. J Arthroplasty 2005; 20: 763-71.
Gray AC, Torrens L, Howie CR, Christie J, Robinson CM. Cognitive function and cerebral emboli after primary hip arthroplasty. Hip Int 2008; 18: 40-5.
Patel R, Stygall J, Harrington J, Newman S, Haddad F. Intra-operative cerebral microembolisation during primary hybrid total hip arthroplasty compared with primary hip resurfacing. Acta Orthop Belg 2009; 75: 671-7.
Patel RV, Stygall J, Harrington J, Newman SP, Haddad FS. Cerebral microembolization during primary total hip arthroplasty and neuropsychologic outcome: a pilot study. Clin Orthop Relat Res 2010; 468: 1621-9.
Koch S, Forteza A, Lavernia C, et al. Cerebral fat microembolism and cognitive decline after hip and knee replacement. Stroke 2007; 38: 1079-81.
Ren P, Li K, Lu X, Xie M. Diagnostic value of transthoracic echocardiography for patent foramen ovale: a meta-analysis. Ultrasound Med Biol 2013; 39: 1743-50.
Jasudavisius A, Arellano R, Martin J, McConnell B, Bainbridge D. A systematic review of transthoracic and transesophageal echocardiography in non-cardiac surgery: implications for point-of-care ultrasound education in the operating room. Can J Anesth 2016; 63: 480-7.
Bainbridge D, Martin J, Ahmad Sabry MH, Craig A, Iglesias I. Orogastric tubes do not improve transesophageal echocardiographic imaging during cardiac surgery: a randomized trial. Can J Anesth 2010; 57: 216-21.
Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990; 113: 941-8.
Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc 2008; 56: 823-30.
Acknowledgements
We sincerely thank Rob Mayer and Nachiket Deshpande for assisting in recruiting patients and collecting and storing the digital images and Lee-Anne Fochesato for administrative assistance.
Conflicts of interest
None declared.
Editorial responsibility
This submission was handled by Dr. Gregory L. Bryson, Deputy Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
Nan Gai was involved with the ethics proposal, study design, patient recruitment, patient follow-up, data collection, and drafting and final approval of the manuscript. Ronit Lavi was involved with the ethics proposal, study design, data collection, review of echocardiogram imaging, and drafting and final approval of the manuscript. Philip M. Jones was involved with the ethics proposal, statistical analysis, and drafting and final approval of the manuscript. Hwa Lee was involved with the ethics proposal, patient recruitment, data collection, patient follow-up, perioperative data collection, and drafting and final approval of the manuscript. Douglas Naudie was involved with the ethics proposal, study design, and drafting and approval of the final manuscript. Daniel Bainbridge was involved with the ethics proposal, study design, data collection, review of echocardiogram imaging, and drafting and final approval of the manuscript.
Funding
Funding for this study was provided by Department of Anesthesia and Perioperative Medicine’s internal research funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gai, N., Lavi, R., Jones, P.M. et al. The use of point-of-care ultrasound to diagnose patent foramen ovale in elective hip and knee arthroplasty patients and its association with postoperative delirium. Can J Anesth/J Can Anesth 65, 619–626 (2018). https://doi.org/10.1007/s12630-018-1073-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-018-1073-7